Abstract
Purpose
The significance of thyroglobulin antibodies (TgAbs) and thyroid peroxidase antibodies (TPOAbs) in Graves’ disease (GD) remains unclear. Therefore, this study aimed to clarify the clinical significance of TgAbs and TPOAbs in GD.
Methods
A total of 442 patients with GD were recruited and divided into four groups based on TgAb and TPOAb positivity. Their clinical parameters and the characteristics of the groups were compared. Cox proportional hazard regression analysis was performed to assess risk factors for GD remission.
Results
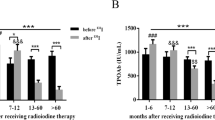
The free triiodothyronine (FT3) level was significantly higher in groups positive for TgAbs and TPOAbs than in the other groups. The FT3 to free thyroxine (FT4) (FT3/FT4) ratio was significantly higher and thyrotropin-stimulating hormone (TSH) receptor antibodies (TRAbs) were significantly lower in the TgAb+/TPOAb− group. Time to FT4 recovery was significantly shorter for groups negative for TPOAbs, whereas time to TSH recovery was significantly longer for groups positive for TPOAbs. Cox proportional hazard regression analysis revealed that TgAb positivity, prolonged treatment duration with antithyroid drugs, and Graves’ ophthalmopathy treated with methylprednisolone were significantly associated with GD remission and that a smoking history, elevated FT3/FT4 ratio, and treatment with propylthiouracil hindered GD remission.
Conclusion
The contributions of TgAbs and TPOAbs to GD pathogenesis differ. Patients positive for TgAbs develop GD with lower TRAb titers and undergo earlier remission than those negative for TgAbs. Patients positive for TPOAbs develop GD with high TRAb titers and need a long time to achieve remission.



Similar content being viewed by others
References
Davis TF, Tomer Y (2021) Thyroid diseases: thyrotoxicosis. In: Braverman LE, Cooper DS, Kopp PA (eds) Werner & Ingbar’s the thyroid: a fundamental and clinical text, 11th edn. Wolters Kluwer, Philadelphia, pp 353–369
Feldt-Rasmussen U (2021) Laboratory measurement of thyroid-related hormones, proteins, and autoantibodies in serum. In: Braverman LE, Cooper DS, Kopp PA (eds) Werner & Ingbar’s the thyroid: a fundamental and clinical text, 11th edn. Wolters Kluwer, Philadelphia, pp 267–299
Fröhlich E, Wahl R (2017) Thyroid Autoimmunity: Role of Anti-thyroid Antibodies in Thyroid and Extra-Thyroidal Diseases. Front Immunol 8:521
Mariotti S, Pisani S, Russova A, Pinchera A (1982) A new solid-phase immunoradiometric assay for anti-thyroglobulin autoantibody. J Endocrinol Invest 5:227–233
Japan Thyroid Association (2010) Guidelines for the Diagnosis of Graves’ Disease. Nankodo Co., Ltd. Tokyo. http://www.japanthyroid.jp/en/guidelines.html#Gra. Accessed 19 Mar 2022
Katahira M, Maeda H, Tosaki T, Segawa S (2009) The human leukocyte antigen class II gene has different contributions to autoimmune type 1 diabetes with or without autoimmune thyroid disease in the Japanese population. Diabetes Res Clin Pract 85:293–297
Diana T, Daiber A, Oelze M, Neumann S, Olivo PD, Kanitz M et al (2018) Stimulatory TSH-Receptor Antibodies and Oxidative Stress in Graves Disease. J Clin Endocrinol Metab 103:3668–3677
Baser H, Can U, Baser S, Yerlikaya FH, Aslan U, Hidayetoglu BT (2015) Assesment of oxidative status and its association with thyroid autoantibodies in patients with euthyroid autoimmune thyroiditis. Endocrine 48:916–923
Ates I, Yilmaz FM, Altay M, Yilmaz N, Berker D, Güler S (2015) The relationship between oxidative stress and autoimmunity in Hashimoto’s thyroiditis. Eur J Endocrinol 173:791–799
Sewerynek J, Wiktorska J, Nowak D, Lewinski A (2000) Methimazole protection against oxidative stress induced by hyperthyroidism in Graves disease. Endocr Regul 34:83–89
Schott M, Eckstein A, Willenberg HS, Nguyen TB, Morgenthaler NG, Scherbaum WA (2007) Improved prediction of relapse of Graves’ thyrotoxicosis by combined determination of TSH receptor and thyroperoxidase antibodies. Horm Metab Res 39:56–61
Hamada N, Ito K, Mimura T, Ishikawa N, Momotani N, Noh J et al (1987) Retrospective reevaluation of the significance of thyroid microsomal antibody in the treatment of Graves’ disease. Acta Endocrinol (Copenh) 114:328–335
Romaldini JH, Bromberg N, Werner RS, Tanaka LM, Rodrigues HF, Werner MC et al (1983) Comparison of effects of high and low dosage regimens of antithyroid drugs in the management of Graves’ hyperthyroidism. J Clin Endocrinol Metab 57:563–570
Guilhem I, Massart C, Poirier JY, Maugendre D (2006) Differential evolution of thyroid peroxidase and thyrotropin receptor antibodies in Graves’ disease: thyroid peroxidase antibody activity reverts to pretreatment level after carbimazole withdrawal. Thyroid 16:1041–1045
Stefanic M, Karner I (2014) Thyroid peroxidase autoantibodies are associated with a lesser likelihood of late reversion to hyperthyroidism after successful non-ablative treatment of Graves’ disease in Croatian patients. J Endocrinol Invest 37:71–77
Hutfless S, Matos P, Talor MV, Caturegli P, Rose NR (2011) Significance of prediagnostic thyroid antibodies in women with autoimmune thyroid disease. J Clin Endocrinol Metab 96:E1466–E1471
Lindgren O, Asp P, Sundlöv A, Tennvall J, Shahida B, Planck T et al (2019) The Effect of Radioiodine Treatment on TRAb, Anti-TPO, and Anti-TG in Graves’ Disease. Eur Thyroid J 8:64–69
Yamaguchi Y, Inukai T, Iwashita A, Nishino M, Yamaguchi T, Shohda Y et al (1990) Changes in thyroid volume during antithyroid drug therapy for Graves’ disease and its relationship to TSH receptor antibodies, TSH and thyroglobulin. Acta Endocrinol (Copenh) 123:411–415
Kuo SW, Huang WS, Hu CA, Liao WK, Fung TC, Wu SY (1994) Effect of thyroxine administration on serum thyrotropin receptor antibody and thyroglobulin levels in patients with Graves’ hyperthyroidism during antithyroid drug therapy. Eur J Endocrinol 131:125–130
Bitton RN, Wexler C (1990) Free triiodothyronine toxicosis: a distinct entity. Am J Med 88:531–533
Figge J, Leinung M, Goodman AD, Izquierdo R, Mydosh T, Gates S (1994) The clinical evaluation of patients with subclinical hyperthyroidism and free triiodothyronine (free T3) toxicosis. Am J Med 96:229–234
Khamisi S, Lundqvist M, Emadi P, Almby K, Ljunggren Ö, Karlsson FA (2021) Serum thyroglobulin is associated with orbitopathy in Graves’ disease. J Endocrinol Invest 44:1905–1911
Goh SY, Ho SC, Seah LL, Fong KS, Khoo DH (2004) Thyroid autoantibody profiles in ophthalmic dominant and thyroid dominant Graves’ disease differ and suggest ophthalmopathy is a multiantigenic disease. Clin Endocrinol (Oxf) 60:600–607
Wojciechowska-Durczynska K, Zygmunt A, Matusiak A, Karbownik-Lewinska M, Lewinski A (2019) Positive TgAbs in patients with Graves’ orbitopathy are associated with lower risk of its active form - preliminary study. Neuro Endocrinol Lett 40:105–110
Eckstein AK, Plicht M, Lax H, Hirche H, Quadbeck B, Mann K et al (2004) Clinical results of anti-inflammatory therapy in Graves’ ophthalmopathy and association with thyroidal autoantibodies. Clin Endocrinol (Oxf) 61:612–618
Bromberg N, Romaldini JH, Werner RS, Sgarbi JA, Werner MC (1992) The evolution of Graves’ ophthalmopathy during treatment with antithyroid drug alone and combined with triiodothyronine. J Endocrinol Invest 15:191–195
Khoo DH, Ho SC, Seah LL, Fong KS, Tai ES, Chee SP et al (1999) The combination of absent thyroid peroxidase antibodies and high thyroid-stimulating immunoglobulin levels in Graves’ disease identifies a group at markedly increased risk of ophthalmopathy. Thyroid 9:1175–1180
Lantz M, Planck T, Asman P, Hallengren B (2014) Increased TRAb and/or low anti-TPO titers at diagnosis of Graves’ disease are associated with an increased risk of developing ophthalmopathy after onset. Exp Clin Endocrinol Diabetes 122:113–117
Lee JH, Park SH, Koh DG, Suh BK (2014) Thyroid peroxidase antibody positivity and triiodothyronine levels are associated with pediatric Graves’ ophthalmopathy. World J Pediatr 10:155–159
Katahira M, Ogata H (2016) Thyroglobulin Autoantibodies Are Associated with Refractoriness to Antithyroid Drug Treatment for Graves’ Disease. Intern Med 55:1519–1524
Takaichi Y, Tamai H, Honda K, Nagai K, Kuma K, Nakagawa T (1989) The significance of antithyroglobulin and antithyroidal microsomal antibodies in patients with hyperthyroidism due to Graves’ disease treated with antithyroidal drugs. J Clin Endocrinol Metab 68:1097–1100
Laurberg P (2006) Remission of Graves’ disease during anti-thyroid drug therapy. Time to reconsider the mechanism? Eur J Endocrinol 155:783–786
Weetman AP (1994) The immunomodulatory effects of antithyroid drugs. Thyroid 4:145–146
Unüvar N, Serter R, Aral Y (1997) The effects of smoking on remission and relapse of Graves’ disease. Clin Endocrinol (Oxf) 46:377–378
Nedrebo BG, Holm PI, Uhlving S, Sorheim JI, Skeie S, Eide GE et al (2002) Predictors of outcome and comparison of different drug regimens for the prevention of relapse in patients with Graves’ disease. Eur J Endocrinol 147:583–589
García-Mayor RV, Álvarez-Vázquez P, Fluiters E, Valverde D, Andrade A (2019) Long-term remission following antithyroid drug withdrawal in patients with Graves’ hyperthyroidism: parameters with prognostic value. Endocrine 63:316–322
Jankauskiene J, Jarusaitiene D (2017) The Influence of Juvenile Graves’ Ophthalmopathy on Graves’ Disease Course. J Ophthalmol 2017:4853905
Eliana F, Suwondo P, Asmarinah A, Harahap A, Djauzi S, Prihartono J et al (2017) The Role of Cytotoxic T-lymphocyte-associated Protein 4 (CTLA-4) Gene, Thyroid Stimulating Hormone Receptor (TSHR) Gene and Regulatory T-cells as Risk Factors for Relapse in Patients with Graves Disease. Acta Med Indones 49:195–204
Liu L, Lu H, Liu Y, Liu C, Xun C (2016) Predicting relapse of Graves’ disease following treatment with antithyroid drugs. Exp Ther Med 11:1453–1458
Anagnostis P, Adamidou F, Polyzos SA, Katergari S, Karathanasi E, Zouli C et al (2013) Predictors of long-term remission in patients with Graves’ disease: a single center experience. Endocrine 44:448–453
Eckstein AK, Lax H, Lösch C, Glowacka D, Plicht M, Mann K et al (2007) Patients with severe Graves’ ophthalmopathy have a higher risk of relapsing hyperthyroidism and are unlikely to remain in remission. Clin Endocrinol (Oxf) 67:607–612
Stenszky V, Balázs C, Kozma L, Rochlitz S, Bear JC, Farid NR (1983) Identification of subsets of patients with Graves’ disease by cluster analysis. Clin Endocrinol (Oxf) 18:335–345
Le Moli R, Malandrino P, Russo M, Lo Giudice F, Frasca F, Belfiore A et al (2020) Corticosteroid Pulse Therapy for Graves’ Ophthalmopathy Reduces the Relapse Rate of Graves’ Hyperthyroidism. Front Endocrinol (Lausanne) 11:367
Marcocci C, Chiovato L, Mariotti S, Pinchera A (1982) Changes of circulating thyroid autoantibody levels during and after the therapy with methimazole in patients with Graves’ disease. J Endocrinol Invest 5:13–19
Chiovato L, Vitti P, Lombardi A, Kohn LD, Pinchera A (1985) Expression of the microsomal antigen on the surface of continuously cultured rat thyroid cells is modulated by thyrotropin. J Clin Endocrinol Metab 61:12–16
Choi YM, Kwak MK, Hong SM, Hong EG (2019) Changes in Thyroid Peroxidase and Thyroglobulin Antibodies Might Be Associated with Graves’ Disease Relapse after Antithyroid Drug Therapy. Endocrinol Metab (Seoul) 34:268–274
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection and analysis were performed by Masahito Katahira, Taku Tsunekawa, and Akira Mizoguchi. The first draft of the manuscript was written by Masahito Katahira, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Ethics Committee of Aichi Prefectural University and Ichinomiya Municipal Hospital and was conducted in accordance with the Declaration of Helsinki.
Consent to participate
Informed consent was obtained from all participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary materials 1:
Supplementary table 1. Clinical characteristics of four groups based on TgAb and TPOAb positivity in the RIA subgroup. Supplementary table 2. Clinical characteristics of four groups based on TgAb and TPOAb positivity in the ECLIA subgroup. Supplementary table 3. Cox proportional hazard multivariate regression analysis using GO as an independent risk factor. Supplementary table 4. Cox proportional hazard multivariate regression analysis using GO treated with MPDS as an independent risk factor.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Katahira, M., Tsunekawa, T., Mizoguchi, A. et al. Clinical significance of thyroglobulin antibodies and thyroid peroxidase antibodies in Graves’ disease: a cross-sectional study. Hormones 22, 253–261 (2023). https://doi.org/10.1007/s42000-023-00437-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42000-023-00437-7