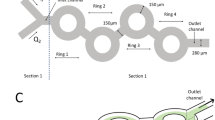
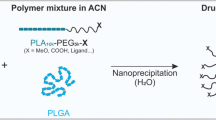
Abstract
Accurate control of core–shell lipopolyplex nanoparticles (LPP NPs) size is crucial for finely adjusting their biomedical performance. However, the synthesis of LPP NPs encounters challenges as two mixing-sensitive processes are involved in the synthesis, rendering precise control over particle size difficult using conventional batch methods. In this study, the formation of the nucleic acid/cationic polymer cores through electrostatic complexation and the subsequent encapsulation by lipid shells via self-assembly were conducted in microreactors, with polyadenylic acid (poly A) and branched polyethylenimine (bPEI) employed as the model system. By assessing the micromixing performance of the microreactors using the Villermaux-Dushman method, the characteristic time scale for electrostatic complexation between poly A and bPEI, as well as the self-assembly of lipids, was determined to be below 1 ms. The Reynolds number, governing micromixing performance, emerged as a crucial factor influencing the sizes of poly A/bPEI cores and LPP NPs. In the kinetic control region, characterized by rapid mixing, the size of poly A/bPEI remained slightly influenced by the N/P molar ratio and volumetric flow rate ratio, irrespective of concentration. The zeta potential, however, was primarily affected by the N/P molar ratio. In the case of LPP NPs, under optimized conditions of anionic lipid molar ratio, the size of LPP NPs was significantly influenced by the composition of lipid shells. This study establishes the foundation for elucidating the structure–activity relationship of LPP NPs.













Similar content being viewed by others
References
Shin MD, Shukla S, Chung YH et al (2020) COVID-19 vaccine development and a potential nanomaterial path forward. Nat Nanotechnol 15:646–655. https://doi.org/10.1038/s41565-020-0737-y
Xiao YF, Tang ZM, Huang XG et al (2022) Emerging mRNA technologies: delivery strategies and biomedical applications. Chem Soc Rev 51:3828–3845. https://doi.org/10.1039/d1cs00617g
Weng YH, Li CH, Yang TR et al (2020) The challenge and prospect of mRNA therapeutics landscape. Biotechnol Adv 40:107534. https://doi.org/10.1016/j.biotechadv.2020.107534
Wang X (2021) Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med 384:1577–1578. https://doi.org/10.1056/NEJMoa2034577
Shi YY, Huang JX, Liu Y et al (2022) Structural and biochemical characteristics of mRNA nanoparticles determine anti–SARS-CoV-2 humoral and cellular immune responses. Sci Adv 8:eabo1827. https://doi.org/10.1126/sciadv.abo1827
Yang R, Deng Y, Huang BY et al (2021) A core-shell structured COVID-19 mRNA vaccine with favorable biodistribution pattern and promising immunity. Signal Transduct Target Ther 6:213. https://doi.org/10.1038/s41392-021-00634-z
Ewe A, Schaper A, Barnert S et al (2014) Storage stability of optimal liposome-polyethylenimine complexes (lipopolyplexes) for DNA or siRNA delivery. Acta Biomater 10:2663–2673. https://doi.org/10.1016/j.actbio.2014.02.037
Wan JW, Yang JM, Wang ZM et al (2023) A single immunization with core–shell structured lipopolyplex mRNA vaccine against rabies induces potent humoral immunity in mice and dogs. Emerg Microbes Infect 12:2270081. https://doi.org/10.1080/22221751.2023.2270081
Geng LJ, Kato N, Kodama Y et al (2023) Influence of lipid composition of messenger RNA-loaded lipid nanoparticles on the protein expression via intratracheal administration in mice. Int J Pharm 637:122896. https://doi.org/10.1016/j.ijpharm.2023.122896
Di JX, Du ZL, Wu KZ et al (2022) Biodistribution and non-linear gene expression of mRNA LNPs affected by delivery route and particle size. Pharm Res 39:105–114. https://doi.org/10.1007/s11095-022-03166-5
Cheng MHY, Leung J, Zhang Y et al (2023) Induction of bleb structures in lipid nanoparticle formulations of mRNA leads to improved transfection potency. Adv Mater 35:2303370. https://doi.org/10.1002/adma.202303370
Wang X, Liu S, Sun YH et al (2023) Preparation of selective organ-targeting (SORT) lipid nanoparticles (LNPs) using multiple technical methods for tissue-specific mRNA delivery. Nat Protoc 18:265–291. https://doi.org/10.1038/s41596-022-00755-x
Hassett KJ, Higgins J, Woods A et al (2021) Impact of lipid nanoparticle size on mRNA vaccine immunogenicity. J Control Release 335:237–246. https://doi.org/10.1016/j.jconrel.2021.05.021
Lam K, Schreiner P, Leung A et al (2023) Optimizing lipid nanoparticles for delivery in primates. Adv Mater 35:2211420. https://doi.org/10.1002/adma.202211420
Linder B, Weirauch U, Ewe A et al (2019) Therapeutic targeting of stat3 using lipopolyplex nanoparticle-formulated siRNA in a syngeneic orthotopic mouse glioma model. Cancers 11:333. https://doi.org/10.3390/cancers11030333
Persano S, Guevara ML, Li ZQ et al (2017) Lipopolyplex potentiates anti-tumor immunity of mRNA-based vaccination. Biomaterials 125:81–89. https://doi.org/10.1016/j.biomaterials.2017.02.019
Du ZX, Munye MM, Tagalakis AD et al (2014) The role of the helper lipid on the DNA transfection efficiency of lipopolyplex formulations. Sci Rep 4:7107. https://doi.org/10.1038/srep07107
Song HM, Wang G, He B et al (2012) Cationic lipid-coated PEI/DNA polyplexes with improved efficiency and reduced cytotoxicity for gene delivery into mesenchymal stem cells. Int J Nanomedicine 7:4637–4648. https://doi.org/10.2147/IJN.S33923
Zhang QY, Ho PY, Tu MJ et al (2018) Lipidation of polyethylenimine-based polyplex increases serum stability of bioengineered RNAi agents and offers more consistent tumoral gene knockdown in vivo. Int J Pharm 547:537–544. https://doi.org/10.1016/j.ijpharm.2018.06.026
Hu YZ, He ZY, Hao Y et al (2019) Kinetic control in assembly of plasmid DNA/polycation complex nanoparticles. ACS Nano 13:10161–10178. https://doi.org/10.1021/acsnano.9b03334
Lombardo D, Kiselev MA (2022) Methods of liposomes preparation: formation and control factors of versatile nanocarriers for biomedical and nanomedicine application. Pharmaceutics 14:543. https://doi.org/10.3390/pharmaceutics14030543
Mitchell MJ, Billingsley MM, Haley RM et al (2020) Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov 20:101–124. https://doi.org/10.1038/s41573-020-0090-8
Chacon WDC, Verruck S, Monteiro AR et al (2023) The mechanism, biopolymers and active compounds for the production of nanoparticles by anti-solvent precipitation: a review. Food Res Int 168:112728. https://doi.org/10.1016/j.foodres.2023.112728
Liu ZK, Yang M, Zhao QK et al (2023) Scale-up of antisolvent precipitation process with ultrasonic microreactors: cavitation patterns, mixing characteristics and application in nanoparticle manufacturing. Chem Eng J 475:146040. https://doi.org/10.1016/j.cej.2023.146040
Johnson BK, Prud’homme RK (2003) Mechanism for rapid self-assembly of block copolymer nanoparticles. Phys Rev Lett 91:118302. https://doi.org/10.1103/PhysRevLett.91.118302
Dinter R, Goette K, Gronke F et al (2023) Development of an automated flow chemistry affinity-based purification process for DNA-encoded chemistry. J Flow Chem. https://doi.org/10.1007/s41981-023-00282-0
Kaisin G, Bovy L, Joyard Y et al (2023) A perspective on automated advanced continuous flow manufacturing units for the upgrading of biobased chemicals toward pharmaceuticals. J Flow Chem 13:77–90. https://doi.org/10.1007/s41981-022-00247-9
Heshmatnezhad F, Nazar ARS (2020) On-chip controlled synthesis of polycaprolactone nanoparticles using continuous-flow microfluidic devices. J Flow Chem 10:533–543. https://doi.org/10.1007/s41981-020-00092-8
Qi TT, Luo GH, Xue HT et al (2023) Continuous heterogeneous synthesis of hexafluoroacetone and its machine learning-assisted optimization. J Flow Chem 13:337–346. https://doi.org/10.1007/s41981-023-00273-1
Ran R, Wang HF, Liu Y et al (2018) Microfluidic self-assembly of a combinatorial library of single- and dual-ligand liposomes for in vitro and in vivo tumor targeting. Eur J Pharm Biopharm 130:1–10. https://doi.org/10.1016/j.ejpb.2018.06.017
Du W, Fu TT, Duan YF et al (2018) Breakup dynamics for droplet formation in shear-thinning fluids in a flow-focusing device. Chem Eng Sci 176:66–76. https://doi.org/10.1016/j.ces.2017.10.019
Liu ZK, Yang M, Yao W et al (2023) Microfluidic ultrasonic cavitation enables versatile and scalable synthesis of monodisperse nanoparticles for biomedical application. Chem Eng Sci 280:119052. https://doi.org/10.1016/j.ces.2023.119052
Balbino TA, Serafin JM, Malfatti-Gasperini AA et al (2016) Microfluidic assembly of pDNA/cationic liposome lipoplexes with high pDNA loading for gene delivery. Langmuir 32:1799–1807. https://doi.org/10.1021/acs.langmuir.5b04177
Koh CG, Zhang XL, Liu SJ et al (2010) Delivery of antisense oligodeoxyribonucleotide lipopolyplex nanoparticles assembled by microfluidic hydrodynamic focusing. J Control Release 141:62–69. https://doi.org/10.1016/j.jconrel.2009.08.019
Commenge J, Falk L (2011) Villermaux-Dushman protocol for experimental characterization of micromixers. Chem Eng Process 50:979–990. https://doi.org/10.1016/j.cep.2011.06.006
Fournier MC, Falk L, Villermaux J (1996) A new parallel competing reaction system for assessing micromixing efficiency-experimental approach. Chem Eng Sci 51:5053–5064. https://doi.org/10.1016/0009-2509(96)00270-9
Soleymani A, Kolehmainen E, Turunen I (2008) Numerical and experimental investigations of liquid mixing in T-type micromixers. Chem Eng J 135:S219–S228. https://doi.org/10.1016/j.cej.2007.07.048
Hoffmann M, Schlüter M, Räbiger N (2006) Experimental investigation of liquid–liquid mixing in T-shaped micro-mixers using μ-LIF and μ-PIV. Chem Eng Sci 61:2968–2976. https://doi.org/10.1016/j.ces.2005.11.029
Bothe D, Stemich C, Warnecke HJ (2006) Fluid mixing in a T-shaped micro-mixer. Chem Eng Sci 61:2950–2958. https://doi.org/10.1016/j.ces.2005.10.060
Yang HJ, Chu GW, Zhang JW et al (2005) Micromixing efficiency in a rotating packed bed: experiments and simulation. Ind Eng Chem Res 44:7730–7737. https://doi.org/10.1021/ie0503646
Yang M, Yang LN, Zheng J et al (2021) Mixing performance and continuous production of nanomaterials in an advanced-flow reactor. Chem Eng J 412:128565. https://doi.org/10.1016/j.cej.2021.128565
Falk L, Commenge JM (2010) Performance comparison of micromixers. Chem Eng Sci 65:405–411. https://doi.org/10.1016/j.ces.2009.05.045
Li SX, Hu YZ, Li A et al (2022) Payload distribution and capacity of mRNA lipid nanoparticles. Nat Commun 13:5561. https://doi.org/10.1038/s41467-022-33157-4
Lokugamage MP, Vanover D, Beyersdorf J et al (2021) Optimization of lipid nanoparticles for the delivery of nebulized therapeutic mRNA to the lungs. Nat Biomed Eng 5:1059–1068. https://doi.org/10.1038/s41551-021-00786-x
Ripoll M, Martin E, Enot M et al (2022) Optimal self-assembly of lipid nanoparticles (LNP) in a ring micromixer. Sci Rep 12:9483. https://doi.org/10.1038/s41598-022-13112-5
Kölbl A, Desplantes V, Grundemann L et al (2013) Kinetic investigation of the Dushman reaction at concentrations relevant to mixing studies in stirred tank reactors. Chem Eng Sci 93:47–54. https://doi.org/10.1016/j.ces.2013.01.067
Wu BC, McClements DJ (2015) Microgels formed by electrostatic complexation of gelatin and OSA starch: potential fat or starch mimetics. Food Hydrocoll 47:87–93. https://doi.org/10.1016/j.foodhyd.2015.01.021
Pal SK, Dhasmana P, Nigam KDP et al (2019) Tuning of particle size in a helical coil reactor. Ind Eng Chem Res 59:3962–3971. https://doi.org/10.1021/acs.iecr.9b04774
Luo LM, Yang M, Chen GW (2022) Continuous synthesis of TiO2-supported noble metal nanoparticles and their application in ammonia borane hydrolysis. Chem Eng Sci 251:117479. https://doi.org/10.1016/j.ces.2022.117479
Clamme JP, Azoulay J, Mély Y (2003) Monitoring of the formation and dissociation of polyethylenimine/DNA complexes by two photon fluorescence correlation spectroscopy. Biophys J 84:1960–1968. https://doi.org/10.1016/s0006-3495(03)75004-8
Ziebarth JD, Kennetz DR, Walker NJ et al (2017) Structural comparisons of PEI/DNA and PEI/siRNA complexes revealed with molecular dynamics simulations. J Phys Chem B 121:1941–1952. https://doi.org/10.1021/acs.jpcb.6b10775
Honoré I, Grosse S, Frison N et al (2005) Transcription of plasmid DNA: influence of plasmid DNA/polyethylenimine complex formation. J Control Release 107:537–546. https://doi.org/10.1016/j.jconrel.2005.06.018
Debus H, Baumhof P, Probst J et al (2010) Delivery of messenger RNA using poly(ethylene imine)–poly(ethylene glycol)-copolymer blends for polyplex formation: biophysical characterization and in vitro transfection properties. J Control Release 148:334–343. https://doi.org/10.1016/j.jconrel.2010.09.007
Kubczak M, Michlewska S, Karimov M et al (2022) Unmodified and tyrosine-modified polyethylenimines as potential carriers for siRNA: biophysical characterization and toxicity. Int J Pharm 614:121468. https://doi.org/10.1016/j.ijpharm.2022.121468
Perche F, Clemencon R, Schulze K et al (2019) Neutral lipopolyplexes for in vivo delivery of conventional and replicative RNA vaccine. Mol Ther Nucleic Acids 17:767–775. https://doi.org/10.1016/j.omtn.2019.07.014
Jovanović AA, Balanč BD, Ota A et al (2018) Comparative effects of cholesterol and β-sitosterol on the liposome membrane characteristics. Eur J Lipid Sci Technol 120:1800039. https://doi.org/10.1002/ejlt.201800039
Kim J, Jozic A, Lin YX et al (2022) Engineering lipid nanoparticles for enhanced intracellular delivery of mRNA through inhalation. ACS Nano 16:14792–14806. https://doi.org/10.1021/acsnano.2c05647
Choi S, Kang B, Yang E et al (2023) Precise control of liposome size using characteristic time depends on solvent type and membrane properties. Sci Rep 13:4728. https://doi.org/10.1038/s41598-023-31895-z
Acknowledgements
The financial support from the National Natural Science Foundation of China (Nos. 22178336, 22208256, 21991103) and Dalian Institute of Chemical Physics Grant (No. DICP I202216) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• The micromixing times of two different microreactors were calculated by incorporation model based on Villermaux-Dushman method.
• The effects of different variables on the formation of poly A/bPEI NPs and LPP NPs was systematically studied under controlled mixing.
• The Reynolds number was found to be a crucial factor influencing the sizes of poly A/bPEI cores and LPP NPs.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, S., Liu, Z., Guo, L. et al. Continuous and size-control synthesis of lipopolyplex nanoparticles enabled by controlled micromixing performance for mRNA delivery. J Flow Chem (2024). https://doi.org/10.1007/s41981-024-00316-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41981-024-00316-1