Abstract
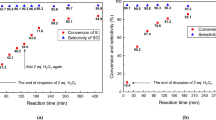
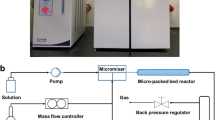
Due to the inherent safety and high mass/heat transfer efficiency of microreactor, the continuous micro-reaction technology has been widely applied in hazardous chemistry recently. In this work, a continuous ozonolysis system based on micro-packed bed reactors (µPBRs) was developed with the synthesis of benzaldehyde as a model reaction. The effects of operating variables (e.g., stirring time, molar ozone/olefin ratio, reaction pressure, reaction temperature and liquid residence time) on the olefins conversion and product distribution were investigated. Based on the experimental results, the optimum reaction conditions are as follows: stirring time 1 h, molar ratio of ozone to olefin 1.2, reaction pressure 0.1 MPa, and the reaction temperature ranging from − 15 to 10℃, as opposed to the low temperature (<-50℃) routinely employed for batch operation. In addition, the full conversion of styrene and a benzaldehyde yield of ~ 93% was observed with the liquid residence time of 3.8–30.8 s. Consequently, the flow ozonolysis technique upon µPBRs allows for a sustainable, safe and efficient approach to oxidize olefins to aldehydes/ketones compared to traditional methods.


Similar content being viewed by others
References
Roughley SD, Jordan AM (2011) The medicinal chemist’s toolbox: an analysis of reactions used in the pursuit of drug candidates[J]. J Med Chem 54(10):3451–3479
Travis BR, Narayan RS, Borhan B (2002) Osmium tetroxide-promoted catalytic oxidative cleavage of olefins: an organometallic ozonolysis[J]. J Am Chem Soc 124(15):3824–3825
Bianga J, Kopplin N, Hülsmann J et al (2020) Rhodium-Catalysed Reductive Amination for the Synthesis of Tertiary Amines[J], vol 362. Advanced Synthesis & Catalysis, pp 4415–4424. 20
Bumler C, Bauer C, Kempe R (2020) The Synthesis of Primary Amines through Reductive Amination Employing an Iron Catalyst[J]. Chemsuschem 13(12):3110–3114
Schäfer C, Ellstrom CJ, Török B (2018) Heterogeneous Catalytic Aqueous Phase Oxidative Cleavage of Styrenes to Benzaldehydes: An Environmentally Benign Alternative to Ozonolysis[J].Topics in Catalysis, (61):643–651
Wang GZ, Li XL, Dai JJ et al (2014) AIBN-catalyzed oxidative cleavage of gem-disubstituted alkenes with O2 as an oxidant[J]. J Org Chem 79(15):7220–7225
Hossain MM, Shyu S-G (2014) Selective copper(II)-catalyzed aerobic oxidative cleavage of aromatic gem-disubstituted alkenes to carbonyl compounds under neutral and mild conditions[J]. Tetrahedron 70(2):251–255
Zou H, Xiao G, Chen K et al (2018) Noble metal-free V2O5/g-C3N4 composites for selective oxidation of olefins using hydrogen peroxide as an oxidant[J]. Dalton Trans 47(38):13565–13572
Rayati S, Moradi D, Nejabat F (2020) Magnetically recoverable porphyrin-based nanocatalysts for the effective oxidation of olefins with hydrogen peroxide: a comparative study[J]. New J Chem 44(44):19385–19392
Kooti M, Afshari M (2012) Magnetic cobalt ferrite nanoparticles as an efficient catalyst for oxidation of alkenes[J]. Scientia Iranica 19(6):1991–1995
Yang D, Zhang C (2001) Ruthenium-catalyzed oxidative cleavage of olefins to aldehydes[J]. J Org Chem 66(14):4814–4818
Allian A (2014) A New Paradigm in Oxidative Cleavage Reaction: The Use of Continuous Reactors To Enable Safe Scale Up of Ozonolysis[J]. ACS Symposium Series, 1181: 353–382
Hida T, Kikuchi J, Kakinuma M et al (2010) Risk Assessment and Safety Evaluation Study for Ozonolysis of β-Pinene: Raw Material of a Novel Prostaglandin D2 Receptor Antagonist S-5751[J], vol 14. Organic Process Research & Development, pp 1485–1489. 6
Ornum SG, V, Champeau RM, Pariza R (2006) Ozonolysis Applications in Drug Synthesis[J]. Chem Rev 106(7):2990–3001
Sukanchan P (2012) An overview of ozonation associated with nano-filtration as an effective procedure in treating dye effluents from textile industries with the help of a bubble column reactor[J]. Res J Chem Environ 16(2):83–86
Wada Y, Schmidt MA, Jensen KF (2006) Flow Distribution and Ozonolysis in Gas-Liquid Multichannel Microreactors[J]. Ind Eng Chem Res 45(24):8036–8042
Bailey PS (1978) Ozonation In Organic Chemistry[M]. Academic Press, New York, pp 25–31
Schiaffo CE, Dussault PH (2008) Ozonolysis in solvent/water mixtures: direct conversion of alkenes to aldehydes and ketones[J]. J Org Chem 73(12):4688–4690
Liang Z, Wei T, Xie J et al (2020) Direct conversion of terminal alkenes to aldehydes via ozonolysis reaction in rotating zigzag bed[J]. J Iran Chem Soc 17(9):2379–2384
Atapalkar RS, Athawale PR, Reddy DS et al (2021) Scalable, sustainable and catalyst-free continuous flow ozonolysis of fatty acids[J]. Green Chem 23(6):2391–2396
Cochran BM (2016) One-pot oxidative cleavage of olefins to synthesize carboxylic acids by a telescoped ozonolysis–oxidation process[J]. Synlett 27(2):245–248
Schwartz C, Raible J, Mott K et al (2006) ‘Reductive ozonolysis’ via a new fragmentation of carbonyl oxides[J]. Tetrahedron 62(46):10747–10752
Schwartz C, Raible J, Mott K et al (2006) Fragmentation of Carbonyl Oxides by N-Oxides: An Improved Approach to Alkene Ozonolysis[J]. Org Lett 8(15):3199–3201
Gaifutdinova EK, Beresnev VV (2002) Synthesis of Ethyl Benzoate by Ozonolysis of Styrene in the Presence of Ethanol[J]. Russ J Appl Chem 75(3):452–454
Silverman JR, Danby AM, Subramaniam B (2019) Intensified ozonolysis of lignins in a spray reactor: insights into product yields and lignin structure[J]. Reaction Chem Eng 4(8):1421–1430
Charnley RW, Fisher T, Johnson J (2012) Pyridine Is an Organocatalyst for the Reductive Ozonolysis of Alkenes[J]. Org Lett 14(9):2242–2245
Kula J (1999) Safer ozonolysis reactions: A compilation of laboratory experience[J], vol 6. Chemical Health & Safety, pp 21–22. 6
Hassan Z, Stahlberger M, Rosenbaum N et al (2020) Criegee Intermediates Beyond Ozonolysis: Recent Synthetic and Mechanistic Insights[J]. Angewandte Chemie
Kendall AJ, Barry JT, Seidenkranz DT et al (2016) Highly efficient biphasic ozonolysis of alkenes using a high-throughput film-shear flow reactor[J]. Tetrahedron Lett 57(12):1342–1345
Losey MW, Schmidt MA, Jensen KF (2001) Microfabricated Multiphase Packed-Bed Reactors: Characterization of Mass Transfer and Reactions[J], vol 40. Industrial & Engineering Chemistry Research, pp 2555–2562. 12
Steinfeldt N, Bentrup U, Jahnisch K (2011) Reaction Mechanism and in Situ ATR Spectroscopic Studies of the 1-Decene Ozonolysis in Micro- and Semibatch Reactors[J]. Ind Eng Chem Res 49(1):72–80
Henry H, Zador M, Fliszar S (1973) A quantitative investigation of the ozonolysis reaction. XVIII. A kinetic study of the ozone attack on phenylethylenes[J]. Can J Chem 51(20):3398–3402
O’brien M, Baxendale IR, Ley SV (2010) Flow ozonolysis using a semipermeable Teflon AF-2400 membrane to effect gas-liquid contact[J]. Org Lett 12(7):1596–1598
Irfan M, Glasnov TN, Kappe CO (2011) Continuous flow ozonolysis in a laboratory scale reactor[J]. Org Lett 13(5):984–987
Hübner S, Bentrup U, Budde U et al (2009) An Ozonolysis – Reduction Sequence for the Synthesis of Pharmaceutical Intermediates in Microstructured Devices[J], vol 13. Organic Process Research & Development, pp 952–960. 5
Roydhouse MD, Ghaini A, Constantinou A et al (2011) Ozonolysis in Flow Using Capillary Reactors[J], vol 15. Organic Process Research & Development, pp 989–996. 5
Figueirêdo MB, Keij F, Hommes A et al (2019) Efficient depolymerization of lignin to biobased chemicals using a two-step approach involving ozonation in a continuous flow microreactor followed by catalytic hydrotreatment[J]. ACS Sustain Chem Eng 7(22):18384–18394
Lee K, Lin H, Jensen KF (2017) Ozonolysis of quinoline and quinoline derivatives in a Corning low flow reactor[J]. Reaction Chem Eng 2(5):696–702
Zhang J, Teixeira AR, Jense KF (2017) Automated measurements of gas-liquid mass transfer in micropacked bed reactors[J]. AIChE J 64(2):564–570
Zhang J, Teixeira AR, Kögl LT et al (2017) Hydrodynamics of gas–liquid flow in micropacked beds: Pressure drop, liquid holdup, and two-phase model[J]. AIChE J 63(10):564–570
Tu JC, Sang L, Cheng H et al (2019) Continuous Hydrogenolysis of N-Diphenylmethyl Groups in a Micropacked-Bed Reactor[J], vol 24. Organic Process Research & Development, pp 59–66. 1
Duan X, Tu J, Teixeira AR et al (2020) An automated flow platform for accurate determination of gas–liquid–solid reaction kinetics[J]. Reaction Chemistry & Engineering, p 5
Glasnov TN, Kappe CO (2010) Toward a Continuous-Flow Synthesis of Boscalid®[J]. Adv Synth Catal 352(17):3089–3097
Al-Rifai N, Galvanin F, Morad M et al (2016) Hydrodynamic effects on three phase micro-packed bed reactor performance–gold–palladium catalysed benzyl alcohol oxidation[J]. Chem Eng Sci 149:129–142
Cao Q, Sang L, Tu JC et al (2020) Rapid degradation of refractory organic pollutants by continuous ozonation in a micro-packed bed reactor[J].Chemosphere, 270(128621)
Cao Q, Lou F, Liu N et al (2021) Continuous Catalytic Ozonation of Antibiotics Using Mn and Cu Oxides on γ-Al2O3 Pellets in a Micropacked Bed Reactor[J], vol 1. ACS ES&T Water, pp 1911–1920. 8
Lundin MD, Danby AM, Akien GR et al (2017) Intensified and safe ozonolysis of fatty acid methyl esters in liquid CO2 in a continuous reactor[J]. AIChE J 63(7):2819–2826
Pappas JJ, Keaveney WP, Gancher E et al (1966) A new and convenient method for converting olefins to aldehydes[J]. Tetrahedron Lett 7(36):4273–4278
Cochran BM, Corbett MT, Correll TL et al (2018) Development of a Commercial Process to Prepare AMG 232 Using A Green Ozonolysis-Pinnick Tandem Transformation[J]. J Org Chem 84(8):4763–4779
Danby AM, Lundin MD, Subramaniam B (2017) Valorization of grass lignins: Swift and selective recovery of pendant aromatic groups with ozone[J]. ACS Sustain Chem Eng 6(1):71–76
Gao W, Du L, Li P et al (2020) A highly efficient synthesis of benzaldehyde by ozonolysis of styrene in a rotating packed bed[J]. Chem Eng Process 158:108166
Panich NM, Ershov BG (2019) Solubility of Ozone in Organic Solvents[J]. Russ J Gen Chem 89(2):185–189
Jordan A, Stoy P, Sneddon HF, Chlorinated Solvents (2020) Their Advantages, Disadvantages, and Alternatives in Organic and Medicinal Chemistry[J]. Chem Rev 121(3):1582–1622
Myasoedova YV, Nazarov IS, Ishmuratov GY (2019) Transformations of Peroxide Products of Alkene Ozonolysis[J]. Russ J Org Chem 55(1):47–73
Ishmuratov GY, Legostaeva YV, Botsman LP et al (2010) Transformations of peroxide products of olefins ozonolysis[J]. Russ J Org Chem 46(11):1593–1621
Yoshioka M (1987) Synthetic studies related to oral β-lactam antibiotics[J]. Pure & Applied Chemistry 59(8):1041–1046
Roydhouse MD, Motherwell WB, Constantinou A et al (2013) Ozonolysis of some complex organic substrates in flow[J]. RSC Adv 3(15):5076–5082
Bailey PS (1958) The Reactions Of Ozone With Organic Compounds[J]. Chem Rev 58(5):927
Criegee R (1975) Mechanism of Ozonolysis[J]. Angewandte Chemie International Edition in English 14(11):745–752
Steinfeldt N, Abdallah R, Dingerdissen U et al (2007) Ozonolysis of Acetic Acid 1-Vinyl-hexyl Ester in a Falling Film Microreactor[J], vol 11. Organic Process Research & Development, pp 1025–1031. 6
Omonov TS, Kharraz E, Foley P et al (2014) The production of biobased nonanal by ozonolysis of fatty acids[J]. RSC Adv 4:53617–53627
Ragan JA, Am Ende DJ, Brenek SJ et al (2003) Safe execution of a large-scale ozonolysis: Preparation of the bisulfite adduct of 2-hydroxyindan-2-carboxaldehyde and its utility in a reductive amination[J], vol 7. Organic Process Research & Development, pp 155–160. 2
Allian AD, Richter SM, Kallemeyn JM et al (2011) The Development of Continuous Process for Alkene Ozonolysis Based on Combined in Situ FTIR, Calorimetry, and Computational Chemistry[J], vol 15. Organic Process Research & Development, pp 91–97. 1
Polterauer D, Roberge D, Hanselmann P et al (2021) Process intensification of ozonolysis reactions using dedicated microstructured reactors[J]. Reaction Chemistry & Engineering
Battilocchio C, Baxendale IR, Biava M et al (2012) A Flow-Based Synthesis of 2-Aminoadamantane-2-carboxylic Acid[J], vol 16. Organic Process Research & Development, pp 798–810. 5
Acknowledgements
We gratefully acknowledge the supports of National Natural Science Foundation of China (21978146, 22022809) and National Key Research and Development Program of China (2019YFA0905100) on this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lou, F., Cao, Q., Zhang, C. et al. Continuous synthesis of benzaldehyde by ozonolysis of styrene in a micro-packed bed reactor. J Flow Chem 12, 307–315 (2022). https://doi.org/10.1007/s41981-022-00220-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41981-022-00220-6