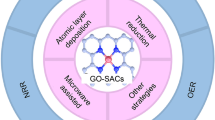
Abstract
Recently, heterogeneous single-atom catalysts (SACs) have attracted enormous attention in electrochemical applications owing to their advantages of high metal utilization, well-defined active sites, tunable selectivity, and excellent activity. To avoid the aggregation of atomically dispersed metal sites, an appropriate support has to be adopted to reduce the surface free energy of catalysts. Graphene with a high surface area, outstanding conductivity, and unique electronic properties has generally been utilized as the substrate for SACs. Moreover, the correlations between metal–support interactions and the electrocatalytic performance at the atomic scale can be studied on graphene-supported single-atom catalyst (G-SAC) nanoplatforms. In this review, we start from an overview of the synthetic methods for G-SACs. Subsequently, several advanced and effective characterization techniques are discussed. Then, we present a comprehensive summary of recent progress in G-SACs for a variety of electrochemical applications. Finally, we present challenges for and an outlook on the development of G-SACs with outstanding catalytic activity, stability, and selectivity.
Graphic Abstract











Copyright © 2016, Nature Publishing Group. b Trends in the oxygen reduction activity via the 2e− and 4e− pathways of different moieties plotted as a function of the OOH* adsorption energy. c ORR pathway for various moieties; d simulated 2e− pathway selectivity as a function of the potential; e chronoamperometric measurement of Co1-NG(O) catalysts in alkali conditions [123]. Reprinted with permission from Ref. [123]. Copyright © 2020, Nature Publishing Group



Copyright © 2018, Nature Publishing Group. c HAADF-STEM image of Ni-doped grapheme [77]. Reprinted with permission from Ref. [77]. Copyright © 2015, Wiley–VCH. d HER stability test of Mo1N1C2 and Pt/C; e free energy diagram for H* adsorption on Mo1N1C2, β-Mo2C, MoN, and N-doped graphene; f calculated DOS of Mo1N1C2 [140]. Reprinted with permission from Ref. [140]. Copyright © 2017, Wiley–VCH. g Linear sweep voltammetry curves of NG, Co–G, Co–NG, and Pt/C in 0.5 M H2SO4 for the HER, where the inset shows an enlarged view near the onset region; h HER stability test of Co–NG in alkali and neutral conditions; i chronoamperometric measurement of Co–NG [52]. Reprinted with permission from Ref. [52]. Copyright © 2015, Nature Publishing Group


Similar content being viewed by others
References
Chu, S., Majumdar, A.: Opportunities and challenges for a sustainable energy future. Nature 488, 294–303 (2012). https://doi.org/10.1038/nature11475
Argyrou, M.C., Christodoulides, P., Kalogirou, S.A.: Energy storage for electricity generation and related processes: technologies appraisal and grid scale applications. Renew. Sustain. Energy Rev. 94, 804–821 (2018). https://doi.org/10.1016/j.rser.2018.06.044
Wang, X.X., Swihart, M.T., Wu, G.: Achievements, challenges and perspectives on cathode catalysts in proton exchange membrane fuel cells for transportation. Nat. Catal. 2, 578–589 (2019). https://doi.org/10.1038/s41929-019-0304-9
Gao, D.F., Arán-Ais, R.M., Jeon, H.S., et al.: Rational catalyst and electrolyte design for CO2 electroreduction towards multicarbon products. Nat. Catal. 2, 198–210 (2019). https://doi.org/10.1038/s41929-019-0235-5
Liu, T., Vivek, J.P., Zhao, E.W., et al.: Current challenges and routes forward for nonaqueous lithium-air batteries. Chem. Rev. 120, 6558–6625 (2020). https://doi.org/10.1021/acs.chemrev.9b00545
Roger, I., Shipman, M.A., Symes, M.D.: Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nat. Rev. Chem. 1, 0003 (2017). https://doi.org/10.1038/s41570-016-0003
Zhang, L., Doyle-Davis, K., Sun, X.L.: Pt-based electrocatalysts with high atom utilization efficiency: from nanostructures to single atoms. Energy Environ. Sci. 12, 492–517 (2019). https://doi.org/10.1039/c8ee02939c
Zhu, Y.Z., Sokolowski, J., Song, X.C., et al.: Engineering local coordination environments of atomically dispersed and heteroatom-coordinated single metal site electrocatalysts for clean energy-conversion. Adv. Energy Mater. 10, 1902844 (2020). https://doi.org/10.1002/aenm.201902844
Mistry, H., Varela, A.S., Kühl, S., et al.: Nanostructured electrocatalysts with tunable activity and selectivity. Nat. Rev. Mater. 1, 16009 (2016). https://doi.org/10.1038/natrevmats.2016.9
Yang, X.F., Wang, A.Q., Qiao, B.T., et al.: Single-atom catalysts: a new frontier in heterogeneous catalysis. Acc. Chem. Res. 46, 1740–1748 (2013). https://doi.org/10.1021/ar300361m
Thirumalai, H., Kitchin, J.R.: Investigating the reactivity of single atom alloys using density functional theory. Top. Catal. 61, 462–474 (2018). https://doi.org/10.1007/s11244-018-0899-0
Qiao, B., Wang, A., Yang, X., et al.: Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat Chem 3, 634–641 (2011). https://doi.org/10.1038/nchem.1095
Li, X.Y., Rong, H.P., Zhang, J.T., et al.: Modulating the local coordination environment of single-atom catalysts for enhanced catalytic performance. Nano Res. 13, 1842–1855 (2020). https://doi.org/10.1007/s12274-020-2755-3
Liu, J., Jiao, M., Lu, L., et al.: High performance platinum single atom electrocatalyst for oxygen reduction reaction. Nat. Commun. 8, 15938 (2017). https://doi.org/10.1038/ncomms15938
Liang, Z.B., Guo, W.H., Zhao, R., et al.: Engineering atomically dispersed metal sites for electrocatalytic energy conversion. Nano Energy 64, 103917 (2019). https://doi.org/10.1016/j.nanoen.2019.103917
Mitchell, S., Vorobyeva, E., Pérez-Ramírez, J.: The multifaceted reactivity of single-atom heterogeneous catalysts. Angew. Chem. Int. Ed. 57, 15316–15329 (2018). https://doi.org/10.1002/anie.201806936
Wang, S.Y., Zhang, L.P., Xia, Z.H., et al.: BCN graphene as efficient metal-free electrocatalyst for the oxygen reduction reaction. Angew. Chem. Int. Ed. 51, 4209–4212 (2012). https://doi.org/10.1002/anie.201109257
Aihara, J.I.: Reduced HOMO–LUMO gap as an index of kinetic stability for polycyclic aromatic hydrocarbons. J. Phys. Chem. A 103, 7487–7495 (1999). https://doi.org/10.1021/jp990092i
Wang, A.Q., Li, J., Zhang, T.: Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2, 65–81 (2018). https://doi.org/10.1038/s41570-018-0010-1
Liu, L., Corma, A.: Metal catalysts for heterogeneous catalysis: from single atoms to nanoclusters and nanoparticles. Chem. Rev. 118, 4981–5079 (2018). https://doi.org/10.1021/acs.chemrev.7b00776
Cui, X.J., Li, W., Ryabchuk, P., et al.: Bridging homogeneous and heterogeneous catalysis by heterogeneous single-metal-site catalysts. Nat. Catal. 1, 385–397 (2018). https://doi.org/10.1038/s41929-018-0090-9
Malta, G., Kondrat, S.A., Freakley, S.J., et al.: Identification of single-site gold catalysis in acetylene hydrochlorination. Science 355, 1399–1403 (2017). https://doi.org/10.1126/science.aal3439
Johansson, M.P., Sundholm, D., Vaara, J.: Au32: a 24-carat golden fullerene. Angewandte Chemie Int. Ed. 43, 2678–2681 (2004). https://doi.org/10.1002/anie.200453986
Zuev, P.S., Sheridan, R.S., Albu, T.V., et al.: Carbon tunneling from a single quantum state. Science 299, 867–870 (2003). https://doi.org/10.1126/science.1079294
Claus, P., Brückner, A., Mohr, C., et al.: Supported gold nanoparticles from quantum dot to mesoscopic size scale: effect of electronic and structural properties on catalytic hydrogenation of conjugated functional groups. J. Am. Chem. Soc. 122, 11430–11439 (2000). https://doi.org/10.1021/ja0012974
Kubo, R.: Electronic properties of metallic fine particles. I. J. Phys. Soc. Jpn. 17, 975–986 (1962). https://doi.org/10.1143/jpsj.17.975
Zhang, L., Si, R., Liu, H., et al.: Atomic layer deposited Pt–Ru dual-metal dimers and identifying their active sites for hydrogen evolution reaction. Nat. Commun. 10, 4936 (2019). https://doi.org/10.1038/s41467-019-12887-y
Cheng, N., Stambula, S., Wang, D., et al.: Platinum single-atom and cluster catalysis of the hydrogen evolution reaction. Nat. Commun. 7, 13638 (2016). https://doi.org/10.1038/ncomms13638
Nakada, K., Ishii, A.: DFT calculation for adatom adsorption on graphene. In: Gong, J.R. (Ed.) Graphene Simulation. InTech, London (2011). https://doi.org/10.5772/20477
Hu, L.B., Hu, X.R., Wu, X.B., et al.: Density functional calculation of transition metal adatom adsorption on graphene. Phys. B Condens. Matter 405, 3337–3341 (2010). https://doi.org/10.1016/j.physb.2010.05.001
Qin, R.X., Liu, P.X., Fu, G., et al.: Strategies for stabilizing atomically dispersed metal catalysts. Small Methods 2, 1700286 (2018). https://doi.org/10.1002/smtd.201700286
Xiong, Y., Sun, W.M., Han, Y.H., et al.: Cobalt single atom site catalysts with ultrahigh metal loading for enhanced aerobic oxidation of ethylbenzene. Nano Res. 14, 2418–2423 (2021). https://doi.org/10.1007/s12274-020-3244-4
Xia, C., Qiu, Y., Xia, Y., et al.: General synthesis of single-atom catalysts with high metal loading using graphene quantum dots. Nat. Chem. 13, 887–894 (2021). https://doi.org/10.1038/s41557-021-00734-x
Gong, X.F., Zhu, J.B., Li, J.Z., et al.: Self-templated hierarchically porous carbon nanorods embedded with atomic Fe-N4 active sites as efficient oxygen reduction electrocatalysts in Zn-air batteries. Adv. Funct. Mater. 31, 2008085 (2021). https://doi.org/10.1002/adfm.202008085
Zhou, Q.Y., Cai, J.J., Zhang, Z., et al.: A gas-phase migration strategy to synthesize atomically dispersed Mn–N–C catalysts for Zn–air batteries. Small Methods 5, 2100024 (2021). https://doi.org/10.1002/smtd.202100024
Ji, S., Chen, Y., Wang, X., et al.: Chemical synthesis of single atomic site catalysts. Chem. Rev. 120, 11900–11955 (2020). https://doi.org/10.1021/acs.chemrev.9b00818
Jang, H., Park, Y.J., Chen, X., et al.: Graphene-based flexible and stretchable electronics. Adv. Mater. 28, 4184–4202 (2016)
Fu, W.Y., Jiang, L., van Geest, E.P., et al.: Sensing at the surface of graphene field-effect transistors. Adv. Mater. 29, 1603610 (2017). https://doi.org/10.1002/adma.201603610
Reina, G., González-Domínguez, J.M., Criado, A., et al.: Promises, facts and challenges for graphene in biomedical applications. Chem. Soc. Rev. 46, 4400–4416 (2017). https://doi.org/10.1039/c7cs00363c
Srinivas, G., Burress, J., Yildirim, T.: Graphene oxide derived carbons (GODCs): synthesis and gas adsorption properties. Energy Environ. Sci. 5, 6453 (2012). https://doi.org/10.1039/c2ee21100a
Ji, L.W., Meduri, P., Agubra, V., et al.: Graphene-based nanocomposites for energy storage. Adv. Energy Mater. 6, 1502159 (2016). https://doi.org/10.1002/aenm.201502159
Novoselov, K.S., Fal′ko, V.I., Colombo, L., et al.: A roadmap for graphene. Nature 490, 192–200 (2012). https://doi.org/10.1038/nature11458
Tan, C., Cao, X., Wu, X.J., et al.: Recent advances in ultrathin two-dimensional nanomaterials. Chem. Rev. 117, 6225–6331 (2017). https://doi.org/10.1021/acs.chemrev.6b00558
Zheng, X.B., Li, P., Dou, S.X., et al.: Non-carbon-supported single-atom site catalysts for electrocatalysis. Energy Environ. Sci. 14, 2809–2858 (2021). https://doi.org/10.1039/d1ee00248a
Guo, J., Yan, X.M., Liu, Q., et al.: The synthesis and synergistic catalysis of iron phthalocyanine and its graphene-based axial complex for enhanced oxygen reduction. Nano Energy 46, 347–355 (2018). https://doi.org/10.1016/j.nanoen.2018.02.026
Wang, X., Sun, G., Routh, P., et al.: Heteroatom-doped graphene materials: syntheses, properties and applications. Chem. Soc. Rev. 43, 7067–7098 (2014). https://doi.org/10.1039/c4cs00141a
Duan, J.J., Chen, S., Jaroniec, M., et al.: Heteroatom-doped graphene-based materials for energy-relevant electrocatalytic processes. ACS Catal. 5, 5207–5234 (2015). https://doi.org/10.1021/acscatal.5b00991
Lin, Z.Y., Waller, G.H., Liu, Y., et al.: 3D Nitrogen-doped graphene prepared by pyrolysis of graphene oxide with polypyrrole for electrocatalysis of oxygen reduction reaction. Nano Energy 2, 241–248 (2013). https://doi.org/10.1016/j.nanoen.2012.09.002
Kim, G., Jhi, S.H., Lim, S., et al.: Effect of vacancy defects in graphene on metal anchoring and hydrogen adsorption. Appl. Phys. Lett. 94, 173102 (2009). https://doi.org/10.1063/1.3126450
Marcano, D.C., Kosynkin, D.V., Berlin, J.M., et al.: Improved synthesis of graphene oxide. ACS Nano 4, 4806–4814 (2010). https://doi.org/10.1021/nn1006368
He, H.Y., Klinowski, J., Forster, M., et al.: A new structural model for graphite oxide. Chem. Phys. Lett. 287, 53–56 (1998). https://doi.org/10.1016/S0009-2614(98)00144-4
Fei, H., Dong, J., Arellano-Jiménez, M.J., et al.: Atomic cobalt on nitrogen-doped graphene for hydrogen generation. Nat. Commun. 6, 8668 (2015). https://doi.org/10.1038/ncomms9668
Jiang, K., Siahrostami, S., Zheng, T.T., et al.: Isolated Ni single atoms in graphene nanosheets for high-performance CO2 reduction. Energy Environ. Sci. 11, 893–903 (2018). https://doi.org/10.1039/c7ee03245e
Fei, H.L., Dong, J.C., Feng, Y.X., et al.: General synthesis and definitive structural identification of MN4C4 single-atom catalysts with tunable electrocatalytic activities. Nat. Catal. 1, 63–72 (2018). https://doi.org/10.1038/s41929-017-0008-y
Zhang, C., Sha, J., Fei, H., et al.: Single-atomic ruthenium catalytic sites on nitrogen-doped graphene for oxygen reduction reaction in acidic medium. ACS Nano 11, 6930–6941 (2017). https://doi.org/10.1021/acsnano.7b02148
Guan, J.Q., Duan, Z.Y., Zhang, F.X., et al.: Water oxidation on a mononuclear manganese heterogeneous catalyst. Nat. Catal. 1, 870–877 (2018). https://doi.org/10.1038/s41929-018-0158-6
Wen, X.D., Duan, Z.Y., Bai, L., et al.: Atomic scandium and nitrogen-codoped graphene for oxygen reduction reaction. J. Power Sources 431, 265–273 (2019). https://doi.org/10.1016/j.jpowsour.2019.126650
Zhao, L., Zhang, Y., Huang, L.B., et al.: Cascade anchoring strategy for general mass production of high-loading single-atomic metal-nitrogen catalysts. Nat. Commun. 10, 1278 (2019). https://doi.org/10.1038/s41467-019-09290-y
Liu, D.B., Wu, C.Q., Chen, S.M., et al.: In situ trapped high-density single metal atoms within graphene: iron-containing hybrids as representatives for efficient oxygen reduction. Nano Res. 11, 2217–2228 (2018). https://doi.org/10.1007/s12274-017-1840-8
Yang, H.B., Hung, S.F., Liu, S., et al.: Atomically dispersed Ni(I) as the active site for electrochemical CO2 reduction. Nat. Energy 3, 140–147 (2018). https://doi.org/10.1038/s41560-017-0078-8
Hou, Y., Qiu, M., Kim, M.G., et al.: Atomically dispersed nickel-nitrogen-sulfur species anchored on porous carbon nanosheets for efficient water oxidation. Nat. Commun. 10, 1392 (2019). https://doi.org/10.1038/s41467-019-09394-5
Jiang, K., Siahrostami, S., Akey, A.J., et al.: Transition-metal single atoms in a graphene shell as active centers for highly efficient artificial photosynthesis. Chem 3, 950–960 (2017). https://doi.org/10.1016/j.chempr.2017.09.014
Yang, L., Cheng, D.J., Xu, H.X., et al.: Unveiling the high-activity origin of single-atom iron catalysts for oxygen reduction reaction. Proc. Natl. Acad. Sci. USA 115, 6626–6631 (2018). https://doi.org/10.1073/pnas.1800771115
Yang, L., Shi, L., Wang, D., et al.: Single-atom cobalt electrocatalysts for foldable solid-state Zn–air battery. Nano Energy 50, 691–698 (2018). https://doi.org/10.1016/j.nanoen.2018.06.023
Deng, D.H., Chen, X.Q., Yu, L., et al.: A single iron site confined in a graphene matrix for the catalytic oxidation of benzene at room temperature. Sci. Adv. 1, e1500462 (2015). https://doi.org/10.1126/sciadv.1500462
Chen, X.Q., Yu, L., Wang, S.H., et al.: Highly active and stable single iron site confined in graphene nanosheets for oxygen reduction reaction. Nano Energy 32, 353–358 (2017). https://doi.org/10.1016/j.nanoen.2016.12.056
Cui, X.J., Xiao, J.P., Wu, Y.H., et al.: A graphene composite material with single cobalt active sites: a highly efficient counter electrode for dye-sensitized solar cells. Angew. Chem. Int. Ed. 55, 6708–6712 (2016). https://doi.org/10.1002/anie.201602097
Lu, J.L., Elam, J.W., Stair, P.C.: Synthesis and stabilization of supported metal catalysts by atomic layer deposition. Acc. Chem. Res. 46, 1806–1815 (2013). https://doi.org/10.1021/ar300229c
Stambula, S., Gauquelin, N., Bugnet, M., et al.: Chemical structure of nitrogen-doped graphene with single platinum atoms and atomic clusters as a platform for the PEMFC electrode. J. Phys. Chem. C 118, 3890–3900 (2014). https://doi.org/10.1021/jp408979h
Sun, S.H., Zhang, G.X., Gauquelin, N., et al.: Single-atom catalysis using Pt/graphene achieved through atomic layer deposition. Sci. Rep. 3, 1775 (2013). https://doi.org/10.1038/srep01775
Yan, H., Cheng, H., Yi, H., et al.: Single-atom Pd1/graphene catalyst achieved by atomic layer deposition: remarkable performance in selective hydrogenation of 1,3-butadiene. J. Am. Chem. Soc. 137, 10484–10487 (2015). https://doi.org/10.1021/jacs.5b06485
Wang, H., Wang, Q., Cheng, Y., et al.: Doping monolayer graphene with single atom substitutions. Nano Lett. 12, 141–144 (2012). https://doi.org/10.1021/nl2031629
Åhlgren, E.H., Kotakoski, J., Krasheninnikov, A.V.: Atomistic simulations of the implantation of low-energy boron and nitrogen ions into graphene. Phys. Rev. B 83, 115424 (2011). https://doi.org/10.1103/physrevb.83.115424
Lehtinen, O., Kotakoski, J., Krasheninnikov, A.V., et al.: Effects of ion bombardment on a two-dimensional target: atomistic simulations of graphene irradiation. Phys. Rev. B 81, 153401 (2010). https://doi.org/10.1103/physrevb.81.153401
Robertson, A.W., Montanari, B., He, K., et al.: Dynamics of single Fe atoms in graphene vacancies. Nano Lett. 13, 1468–1475 (2013). https://doi.org/10.1021/nl304495v
Fei, H.L., Dong, J.C., Wan, C.Z., et al.: Microwave-assisted rapid synthesis of graphene-supported single atomic metals. Adv. Mater. 30, 1802146 (2018). https://doi.org/10.1002/adma.201802146
Qiu, H.J., Ito, Y., Cong, W.T., et al.: Nanoporous graphene with single-atom nickel dopants: an efficient and stable catalyst for electrochemical hydrogen production. Angew. Chem. Int. Ed. 54, 14031–14035 (2015). https://doi.org/10.1002/anie.201507381
Maschmeyer, T., Rey, F., Sankar, G., et al.: Heterogeneous catalysts obtained by grafting metallocene complexes onto mesoporous silica. Nature 378, 159–162 (1995). https://doi.org/10.1038/378159a0
Liu, J.Y.: Aberration-corrected scanning transmission electron microscopy in single-atom catalysis: probing the catalytically active centers. Chin. J. Catal. 38, 1460–1472 (2017). https://doi.org/10.1016/S1872-2067(17)62900-0
Ogino, I.: X-ray absorption spectroscopy for single-atom catalysts: critical importance and persistent challenges. Chin. J. Catal. 38, 1481–1488 (2017). https://doi.org/10.1016/S1872-2067(17)62880-8
Haider, M., Rose, H., Uhlemann, S., et al.: A spherical-aberration-corrected 200 kV transmission electron microscope. Ultramicroscopy 75, 53–60 (1998). https://doi.org/10.1016/S0304-3991(98)00048-5
Haider, M., Uhlemann, S., Schwan, E., et al.: Electron microscopy image enhanced. Nature 392, 768–769 (1998). https://doi.org/10.1038/33823
Krivanek, O.L., Dellby, N., Lupini, A.R.: Towards sub-Å electron beams. Ultramicroscopy 78, 1–11 (1999). https://doi.org/10.1016/S0304-3991(99)00013-3
Lacroix, L.M., Arenal, R., Viau, G.: Dynamic HAADF-STEM observation of a single-atom chain as the transient state of gold ultrathin nanowire breakdown. J. Am. Chem. Soc. 136, 13075–13077 (2014). https://doi.org/10.1021/ja507728j
Zheng, J.K., Luo, R.C., Zeng, X.Q., et al.: Nano-scale precipitation and phase growth in Mg-Gd binary alloy: an atomic-scale investigation using HAADF-STEM. Mater. Des. 137, 316–324 (2018). https://doi.org/10.1016/j.matdes.2017.10.042
Jia, Q.Y., Liu, E.S., Jiao, L., et al.: X-ray absorption spectroscopy characterizations on PGM-free electrocatalysts: justification, advantages, and limitations. Adv. Mater. 31, 1805157 (2019). https://doi.org/10.1002/adma.201805157
Rehr, J.J., Albers, R.C.: Theoretical approaches to X-ray absorption fine structure. Rev. Mod. Phys. 72, 621–654 (2000). https://doi.org/10.1103/revmodphys.72.621
Funke, H., Scheinost, A.C., Chukalina, M.: Wavelet analysis of extended X-ray absorption fine structure data. Phys. Rev. B 71, 094110 (2005). https://doi.org/10.1103/physrevb.71.094110
Li, J.K., Ghoshal, S., Liang, W.T., et al.: Structural and mechanistic basis for the high activity of Fe–N–C catalysts toward oxygen reduction. Energy Environ. Sci. 9, 2418–2432 (2016). https://doi.org/10.1039/c6ee01160h
Zitolo, A., Ranjbar-Sahraie, N., Mineva, T., et al.: Identification of catalytic sites in cobalt-nitrogen-carbon materials for the oxygen reduction reaction. Nat. Commun. 8, 957 (2017). https://doi.org/10.1038/s41467-017-01100-7
Zagal, J.H., Koper, M.T.M.: Reactivity descriptors for the activity of molecular MN4 catalysts for the oxygen reduction reaction. Angew. Chem. Int. Ed. 55, 14510–14521 (2016). https://doi.org/10.1002/anie.201604311
Cao, L.L., Luo, Q.Q., Liu, W., et al.: Identification of single-atom active sites in carbon-based cobalt catalysts during electrocatalytic hydrogen evolution. Nat. Catal. 2, 134–141 (2019). https://doi.org/10.1038/s41929-018-0203-5
Miller, M.K., Kelly, T.F., Rajan, K., et al.: The future of atom probe tomography. Mater. Today 15, 158–165 (2012). https://doi.org/10.1016/S1369-7021(12)70069-X
Kelly, T.F., Miller, M.K.: Atom probe tomography. Rev. Sci. Instruments 78, 031101 (2007). https://doi.org/10.1063/1.2709758
Miller, M.K., Kenik, E.A.: Atom probe tomography: a technique for nanoscale characterization. Microsc. Microanal. 10, 336–341 (2004). https://doi.org/10.1017/s1431927604040577
Kramm, U.I., Ni, L.M., Wagner, S.: 57Fe Mössbauer spectroscopy characterization of electrocatalysts. Adv. Mater. 31, 1805623 (2019). https://doi.org/10.1002/adma.201805623
Zboril, R., Mashlan, M., Petridis, D.: Iron(III) oxides from thermal processes–synthesis, structural and magnetic properties, Mössbauer spectroscopy characterization, and applications. Chem. Mater. 14, 969–982 (2002). https://doi.org/10.1021/cm0111074
Dumesic, J.A., Topsøe, H.: Mössbauer spectroscopy applications to heterogeneous catalysis. Adv. Catal. 26, 121–246 (1977). https://doi.org/10.1016/S0360-0564(08)60071-1
Zitolo, A., Goellner, V., Armel, V., et al.: Identification of catalytic sites for oxygen reduction in iron-and nitrogen-doped graphene materials. Nat. Mater. 14, 937–942 (2015). https://doi.org/10.1038/nmat4367
Kramm, U.I., Herranz, J., Larouche, N., et al.: Structure of the catalytic sites in Fe/N/C-catalysts for O2− reduction in PEM fuel cells. Phys. Chem. Chem. Phys. 14, 11673–11688 (2012). https://doi.org/10.1039/c2cp41957b
Schulenburg, H., Stankov, S., Schünemann, V., et al.: Catalysts for the oxygen reduction from heat-treated iron(III) tetramethoxyphenylporphyrin chloride: structure and stability of active sites. J. Phys. Chem. B 107, 9034–9041 (2003). https://doi.org/10.1021/jp030349j
Li, J.Z., Zhang, H.G., Samarakoon, W., et al.: Thermally driven structure and performance evolution of atomically dispersed FeN4 sites for oxygen reduction. Angew. Chem. Int. Ed. 58, 18971–18980 (2019). https://doi.org/10.1002/anie.201909312
Zhang, N., Zhou, T.P., Chen, M.L., et al.: High-purity pyrrole-type FeN4 sites as a superior oxygen reduction electrocatalyst. Energy Environ. Sci. 13, 111–118 (2020). https://doi.org/10.1039/c9ee03027a
Liu, D., Li, J.C., Ding, S.C., et al.: 2D single-atom catalyst with optimized iron sites produced by thermal melting of metal-organic frameworks for oxygen reduction reaction. Small Methods 4, 1900827 (2020). https://doi.org/10.1002/smtd.201900827
Byon, H.R., Suntivich, J., Shao-Horn, Y.: Graphene-based non-noble-metal catalysts for oxygen reduction reaction in acid. Chem. Mater. 23, 3421–3428 (2011). https://doi.org/10.1021/cm2000649
Zhong, L.X., Li, S.Z.: Unconventional oxygen reduction reaction mechanism and scaling relation on single-atom catalysts. ACS Catal. 10, 4313–4318 (2020). https://doi.org/10.1021/acscatal.0c00815
Kulkarni, A., Siahrostami, S., Patel, A., et al.: Understanding catalytic activity trends in the oxygen reduction reaction. Chem. Rev. 118, 2302–2312 (2018). https://doi.org/10.1021/acs.chemrev.7b00488
Rossmeisl, J., Logadottir, A., Nørskov, J.K.: Electrolysis of water on (oxidized) metal surfaces. Chem. Phys. 319, 178–184 (2005). https://doi.org/10.1016/j.chemphys.2005.05.038
Kang, S.F., Chang, H.M.: Coagulation of textile secondary effluents with Fenton’s reagent. Water Sci. Technol. 36, 215–222 (1997). https://doi.org/10.2166/wst.1997.0450
Walling, C.: Fenton’s reagent revisited. Acc. Chem. Res. 8, 125–131 (1975). https://doi.org/10.1021/ar50088a003
Jiang, H.L., He, Q., Wang, C.D., et al.: Definitive structural identification toward molecule-type sites within 1D and 2D carbon-based catalysts. Adv. Energy Mater. 8, 1800436 (2018). https://doi.org/10.1002/aenm.201800436
Wang, X.X., Prabhakaran, V., He, Y.H., et al.: Iron-free cathode catalysts for proton-exchange-membrane fuel cells: cobalt catalysts and the peroxide mitigation approach. Adv. Mater. 31, 1805126 (2019). https://doi.org/10.1002/adma.201805126
Wu, H.H., Li, H.B., Zhao, X.F., et al.: Highly doped and exposed Cu(I)–N active sites within graphene towards efficient oxygen reduction for zinc–air batteries. Energy Environ. Sci. 9, 3736–3745 (2016). https://doi.org/10.1039/c6ee01867j
Han, G.K., Zheng, Y., Zhang, X., et al.: High loading single-atom Cu dispersed on graphene for efficient oxygen reduction reaction. Nano Energy 66, 104088 (2019). https://doi.org/10.1016/j.nanoen.2019.104088
Wang, J., Wang, K., Wang, F.B., et al.: Bioinspired copper catalyst effective for both reduction and evolution of oxygen. Nat. Commun. 5, 5285 (2014). https://doi.org/10.1038/ncomms6285
Ye, R.Q., Dong, J.C., Wang, L.Q., et al.: Manganese deception on graphene and implications in catalysis. Carbon 132, 623–631 (2018). https://doi.org/10.1016/j.carbon.2018.02.082
Li, J.Z., Chen, M.J., Cullen, D.A., et al.: Atomically dispersed manganese catalysts for oxygen reduction in proton-exchange membrane fuel cells. Nat. Catal. 1, 935–945 (2018). https://doi.org/10.1038/s41929-018-0164-8
Siahrostami, S., Verdaguer-Casadevall, A., Karamad, M., et al.: Enabling direct H2O2 production through rational electrocatalyst design. Nat. Mater. 12, 1137–1143 (2013). https://doi.org/10.1038/nmat3795
Xia, C., Xia, Y., Zhu, P., et al.: Direct electrosynthesis of pure aqueous H2O2 solutions up to 20% by weight using a solid electrolyte. Science 366, 226–231 (2019). https://doi.org/10.1126/science.aay1844
Choi, C.H., Kwon, H.C., Yook, S., et al.: Hydrogen peroxide synthesis via enhanced two-electron oxygen reduction pathway on carbon-coated Pt surface. J. Phys. Chem. C 118, 30063–30070 (2014). https://doi.org/10.1021/jp5113894
Zheng, Y., Jiao, Y., Ge, L., et al.: Two-step boron and nitrogen doping in graphene for enhanced synergistic catalysis. Angew. Chem. Int. Ed. 52, 3110–3116 (2013). https://doi.org/10.1002/anie.201209548
Choi, C.H., Kim, M., Kwon, H.C., et al.: Tuning selectivity of electrochemical reactions by atomically dispersed platinum catalyst. Nat. Commun. 7, 10922 (2016). https://doi.org/10.1038/ncomms10922
Jung, E., Shin, H., Lee, B.H., et al.: Atomic-level tuning of Co-N-C catalyst for high-performance electrochemical H2O2 production. Nat. Mater. 19, 436–442 (2020). https://doi.org/10.1038/s41563-019-0571-5
Jiao, Y., Zheng, Y., Jaroniec, M., et al.: Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 44, 2060–2086 (2015). https://doi.org/10.1039/c4cs00470a
Suen, N.T., Hung, S.F., Quan, Q., et al.: Electrocatalysis for the oxygen evolution reaction: recent development and future perspectives. Chem. Soc. Rev. 46, 337–365 (2017). https://doi.org/10.1039/c6cs00328a
Gao, G.P., Jiao, Y., Waclawik, E.R., et al.: Single atom (Pd/Pt) supported on graphitic carbon nitride as an efficient photocatalyst for visible-light reduction of carbon dioxide. J. Am. Chem. Soc. 138, 6292–6297 (2016). https://doi.org/10.1021/jacs.6b02692
Zhang, L.Z., Jia, Y., Gao, G.P., et al.: Graphene defects trap atomic Ni species for hydrogen and oxygen evolution reactions. Chem 4, 285–297 (2018). https://doi.org/10.1016/j.chempr.2017.12.005
Tavakkoli, M., Flahaut, E., Peljo, P., et al.: Mesoporous single-atom-doped graphene–carbon nanotube hybrid: synthesis and tunable electrocatalytic activity for oxygen evolution and reduction reactions. ACS Catal. 10, 4647–4658 (2020). https://doi.org/10.1021/acscatal.0c00352
Wu, J.B., Zhou, H., Li, Q., et al.: Densely populated isolated single Co–N site for efficient oxygen electrocatalysis. Adv. Energy Mater. 9, 1900149 (2019). https://doi.org/10.1002/aenm.201900149
Bai, L., Duan, Z.Y., Wen, X.D., et al.: Highly dispersed ruthenium-based multifunctional electrocatalyst. ACS Catal. 9, 9897–9904 (2019). https://doi.org/10.1021/acscatal.9b03514
Fernando, A., Aikens, C.M.: Theoretical investigation of water oxidation catalysis by a model manganese cubane complex. J. Phys. Chem. C 120, 21148–21161 (2016). https://doi.org/10.1021/acs.jpcc.6b03029
Britt, R.D., Suess, D.L.M., Stich, T.A.: An Mn(V)–oxo role in splitting water? Proc. Natl. Acad. Sci. USA 112, 5265–5266 (2015). https://doi.org/10.1073/pnas.1505223112
Busch, M., Ahlberg, E., Panas, I.: Electrocatalytic oxygen evolution from water on a Mn(III–V) dimer model catalyst: a DFT perspective. Phys. Chem. Chem. Phys. 13, 15069 (2011). https://doi.org/10.1039/c0cp02132f
Shaffer, D.W., Xie, Y., Concepcion, J.J.: O–O bond formation in ruthenium-catalyzed water oxidation: single-site nucleophilic attack vs. O–O radical coupling. Chem. Soc. Rev. 46, 6170–6193 (2017). https://doi.org/10.1039/c7cs00542c
Li, X.C., Li, J., Siegbahn, P.E.M.: A theoretical study of the recently suggested MnVII mechanism for O–O bond formation in photosystem II. J. Phys. Chem. A 124, 8011–8018 (2020). https://doi.org/10.1021/acs.jpca.0c05135
Zhang, Q.Q., Duan, Z.Y., Li, M., et al.: Atomic cobalt catalysts for the oxygen evolution reaction. Chem. Commun. 56, 794–797 (2020). https://doi.org/10.1039/c9cc09007j
Ye, S.H., Luo, F.Y., Zhang, Q.L., et al.: Highly stable single Pt atomic sites anchored on aniline-stacked graphene for hydrogen evolution reaction. Energy Environ. Sci. 12, 1000–1007 (2019). https://doi.org/10.1039/c8ee02888e
Zhang, Z., Chen, Y., Zhou, L., et al.: The simplest construction of single-site catalysts by the synergism of micropore trapping and nitrogen anchoring. Nat. Commun. 10, 1657 (2019). https://doi.org/10.1038/s41467-019-09596-x
Liu, D.B., Li, X.Y., Chen, S.M., et al.: Atomically dispersed platinum supported on curved carbon supports for efficient electrocatalytic hydrogen evolution. Nat. Energy 4, 512–518 (2019). https://doi.org/10.1038/s41560-019-0402-6
Chen, W.X., Pei, J.J., He, C.T., et al.: Rational design of single molybdenum atoms anchored on N-doped carbon for effective hydrogen evolution reaction. Angew. Chem. Int. Ed. 56, 16086–16090 (2017). https://doi.org/10.1002/anie.201710599
Kortlever, R., Shen, J., Schouten, K.J.P., et al.: Catalysts and reaction pathways for the electrochemical reduction of carbon dioxide. J. Phys. Chem. Lett. 6, 4073–4082 (2015). https://doi.org/10.1021/acs.jpclett.5b01559
Peterson, A.A., Nørskov, J.K.: Activity descriptors for CO2 electroreduction to methane on transition-metal catalysts. J. Phys. Chem. Lett. 3, 251–258 (2012). https://doi.org/10.1021/jz201461p
Wang, Z.X., Zhao, J.X., Cai, Q.H.: CO2 electroreduction performance of a single transition metal atom supported on porphyrin-like graphene: a computational study. Phys. Chem. Chem. Phys. 19, 23113–23121 (2017). https://doi.org/10.1039/c7cp04299j
Back, S., Lim, J., Kim, N.Y., et al.: Single-atom catalysts for CO2 electroreduction with significant activity and selectivity improvements. Chem. Sci. 8, 1090–1096 (2017). https://doi.org/10.1039/c6sc03911a
Peng, B.S., Liu, H.T., Liu, Z.Y., et al.: Toward rational design of single-atom catalysts. J. Phys. Chem. Lett. 12, 2837–2847 (2021). https://doi.org/10.1021/acs.jpclett.1c00049
Raciti, D., Wang, C.: Recent advances in CO2 reduction electrocatalysis on copper. ACS Energy Lett. 3, 1545–1556 (2018). https://doi.org/10.1021/acsenergylett.8b00553
Vorobyeva, E., Gerken, V.C., Mitchell, S., et al.: Activation of copper species on carbon nitride for enhanced activity in the arylation of amines. ACS Catal. 10, 11069–11080 (2020). https://doi.org/10.1021/acscatal.0c03164
Xu, C.C., Zhi, X., Vasileff, A., et al.: Highly selective two-electron electrocatalytic CO2 reduction on single-atom Cu catalysts. Small Struct. 2, 2000058 (2021). https://doi.org/10.1002/sstr.202000058
Yang, H.P., Wu, Y., Li, G.D., et al.: Scalable production of efficient single-atom copper decorated carbon membranes for CO2 electroreduction to methanol. J. Am. Chem. Soc. 141, 12717–12723 (2019). https://doi.org/10.1021/jacs.9b04907
Karapinar, D., Huan, N.T., Ranjbar Sahraie, N., et al.: Electroreduction of CO2 on single-site copper-nitrogen-doped carbon material: selective formation of ethanol and reversible restructuration of the metal sites. Angew. Chem. Int. Ed. 58, 15098–15103 (2019). https://doi.org/10.1002/anie.201907994
Zheng, Y., Vasileff, A., Zhou, X.L., et al.: Understanding the roadmap for electrochemical reduction of CO2 to multi-carbon oxygenates and hydrocarbons on copper-based catalysts. J. Am. Chem. Soc. 141, 7646–7659 (2019). https://doi.org/10.1021/jacs.9b02124
Bi, W.T., Li, X.G., You, R., et al.: Surface immobilization of transition metal ions on nitrogen-doped graphene realizing high-efficient and selective CO2 reduction. Adv. Mater. 30, 1706617 (2018). https://doi.org/10.1002/adma.201706617
Zhang, C.H., Yang, S.Z., Wu, J.J., et al.: Electrochemical CO2 reduction with atomic iron-dispersed on nitrogen-doped graphene. Adv. Energy Mater. 8, 1703487 (2018). https://doi.org/10.1002/aenm.201703487
He, H.Y., Jagvaral, Y.: Electrochemical reduction of CO2 on graphene supported transition metals: towards single atom catalysts. Phys. Chem. Chem. Phys. 19, 11436–11446 (2017). https://doi.org/10.1039/c7cp00915a
Su, P.P., Iwase, K., Nakanishi, S., et al.: Nickel-nitrogen-modified graphene: an efficient electrocatalyst for the reduction of carbon dioxide to carbon monoxide. Small 12, 6083–6089 (2016). https://doi.org/10.1002/smll.201602158
Varela, A.S., Ranjbar Sahraie, N., Steinberg, J., et al.: Metal-doped nitrogenated carbon as an efficient catalyst for direct CO2 electroreduction to CO and hydrocarbons. Angew. Chem. Int. Ed. 54, 10758–10762 (2015). https://doi.org/10.1002/anie.201502099
Foster, S.L., Bakovic, S.I.P., Duda, R.D., et al.: Catalysts for nitrogen reduction to ammonia. Nat. Catal. 1, 490–500 (2018). https://doi.org/10.1038/s41929-018-0092-7
van der Ham, C.J., Koper, M.T., Hetterscheid, D.G.: Challenges in reduction of dinitrogen by proton and electron transfer. Chem. Soc. Rev. 43, 5183–5191 (2014). https://doi.org/10.1039/c4cs00085d
Klerke, A., Christensen, C.H., Nørskov, J.K., et al.: Ammonia for hydrogen storage: challenges and opportunities. J. Mater. Chem. 18, 2304 (2008). https://doi.org/10.1039/b720020j
Deng, J., Iñiguez, J.A., Liu, C.: Electrocatalytic nitrogen reduction at low temperature. Joule 2, 846–856 (2018). https://doi.org/10.1016/j.joule.2018.04.014
Tang, S., Dang, Q., Liu, T., et al.: Realizing a not-strong-not-weak polarization electric field in single-atom catalysts sandwiched by boron nitride and graphene sheets for efficient nitrogen fixation. J. Am. Chem. Soc. 142, 19308–19315 (2020)
Huang, Y., Yang, T.T., Yang, L., et al.: Graphene–boron nitride hybrid-supported single Mo atom electrocatalysts for efficient nitrogen reduction reaction. J. Mater. Chem. A 7, 15173–15180 (2019). https://doi.org/10.1039/c9ta02947h
Tian, X.L., Wang, L.J., Deng, P.L., et al.: Research advances in unsupported Pt-based catalysts for electrochemical methanol oxidation. J. Energy Chem. 26, 1067–1076 (2017). https://doi.org/10.1016/j.jechem.2017.10.009
Liu, M.M., Zhang, R.Z., Chen, W.: Graphene-supported nanoelectrocatalysts for fuel cells: synthesis, properties, and applications. Chem. Rev. 114, 5117–5160 (2014). https://doi.org/10.1021/cr400523y
Kakati, N., Maiti, J., Lee, S.H., et al.: Anode catalysts for direct methanol fuel cells in acidic media: do we have any alternative for Pt or Pt–Ru? Chem. Rev. 114, 12397–12429 (2014). https://doi.org/10.1021/cr400389f
Li, M.F., Duanmu, K.N., Wan, C.Z., et al.: Single-atom tailoring of platinum nanocatalysts for high-performance multifunctional electrocatalysis. Nat. Catal. 2, 495–503 (2019). https://doi.org/10.1038/s41929-019-0279-6
Jin, L.J., Xu, H., Chen, C.Y., et al.: Three-dimensional PdCuM (M = Ru, Rh, Ir) trimetallic alloy nanosheets for enhancing methanol oxidation electrocatalysis. ACS Appl. Mater. Interfaces 11, 42123–42130 (2019). https://doi.org/10.1021/acsami.9b13557
Huang, L., Zhang, X.P., Wang, Q.Q., et al.: Shape-control of Pt–Ru nanocrystals: tuning surface structure for enhanced electrocatalytic methanol oxidation. J. Am. Chem. Soc. 140, 1142–1147 (2018). https://doi.org/10.1021/jacs.7b12353
Duchesne, P.N., Li, Z.Y., Deming, C.P., et al.: Golden single-atomic-site platinum electrocatalysts. Nat. Mater. 17, 1033–1039 (2018). https://doi.org/10.1038/s41563-018-0167-5
Yang, S., Kim, J., Tak, Y.J., Soon, A., Lee, H.: Single-atom catalyst of platinum supported on titanium nitride for selective electrochemical reactions. Angew. Chem. Int. Ed. 128, 2098–2102 (2016). https://doi.org/10.1002/anie.201509241
Yang, S., Lee, H.: Atomically dispersed platinum on gold nano-octahedra with high catalytic activity on formic acid oxidation. ACS Catal. 3, 437–443 (2013). https://doi.org/10.1021/cs300809j
Chen, Q.S., Zhou, Z.Y., Vidal-Iglesias, F.J., et al.: Significantly enhancing catalytic activity of tetrahexahedral Pt nanocrystals by Bi adatom decoration. J. Am. Chem. Soc. 133, 12930–12933 (2011). https://doi.org/10.1021/ja2042029
Neurock, M., Janik, M., Wieckowski, A.: A first principles comparison of the mechanism and site requirements for the electrocatalytic oxidation of methanol and formic acid over Pt. Faraday Discuss. 140, 363–378 (2008). https://doi.org/10.1039/B804591G
Xiong, Y., Dong, J.C., Huang, Z.Q., et al.: Single-atom Rh/N-doped carbon electrocatalyst for formic acid oxidation. Nat. Nanotechnol. 15, 390–397 (2020). https://doi.org/10.1038/s41565-020-0665-x
Zheng, T.T., Jiang, K., Ta, N., et al.: Large-scale and highly selective CO2 electrocatalytic reduction on nickel single-atom catalyst. Joule 3, 265–278 (2019). https://doi.org/10.1016/j.joule.2018.10.015
He, X.H., Deng, Y.C., Zhang, Y., et al.: Mechanochemical kilogram-scale synthesis of noble metal single-atom catalysts. Cell Rep. Phys. Sci. 1, 100004 (2020). https://doi.org/10.1016/j.xcrp.2019.100004
Weng, L.C., Bell, A.T., Weber, A.Z.: Modeling gas-diffusion electrodes for CO2 reduction. Phys. Chem. Chem. Phys. 20, 16973–16984 (2018). https://doi.org/10.1039/c8cp01319e
Ji, M.B., Wei, Z.D.: A review of water management in polymer electrolyte membrane fuel cells. Energies 2, 1057–1106 (2009). https://doi.org/10.3390/en20401057
Bai, L., Duan, Z.Y., Wen, X.D., et al.: Atomically dispersed manganese-based catalysts for efficient catalysis of oxygen reduction reaction. Appl. Catal. B Environ. 257, 117930 (2019). https://doi.org/10.1016/j.apcatb.2019.117930
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant Nos. 21673064, 51802059, 21905070 and 21503059), State Grid Heilongjiang Electric Power Co., Ltd., Technology Project Funding (52243719004s), China Postdoctoral Science Foundation (Grant Nos. 2018M631938, 2018T110307 and 2017M621284), Heilongjiang Postdoctoral Fund (LBH-Z17074 and LBH-Z18066), and Fundamental Research Funds for the Central Universities (Grant Nos. HIT. NSRIF. 2019040 and 2019041).
Author information
Authors and Affiliations
Contributions
The idea for the article was contributed by Yunkun Dai and Lei Zhao. The literature search and data analysis were performed by Fanrong Kong, Xuehan Tai, Yunlong Zhang, Bing Liu, Jiajun Cai, Xiaofei Gong, Yunfei Xia, Hongda Zhang, and Lin Li. The revision of the manuscript was completed by Yunkun Dai, Pan Guo, Bo Liu, and Lei Zhao. The first draft of the manuscript was written by Yunkun Dai. Lei Zhao,Xulei Sui, and Zhenbo Wang critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Dai, Y., Kong, F., Tai, X. et al. Advances in Graphene-Supported Single-Atom Catalysts for Clean Energy Conversion. Electrochem. Energy Rev. 5 (Suppl 2), 22 (2022). https://doi.org/10.1007/s41918-022-00142-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41918-022-00142-w