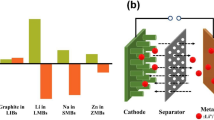
Abstract
Metal anodes (e.g., lithium, sodium and zinc metal anodes) based on a unique plating/stripping mechanism have been well recognized as the most promising anodes for next-generation high-energy metal batteries owing to their superior theoretical specific capacities and low redox potentials. However, realizing full utilization and the theoretical capacity of metal anodes remains challenging because of their high reactivity, poor reversibility, and nonplanar metal evolution patterns, which lead to irreversible loss of active metals and the electrolyte. To minimize the above issues, excess metal sources and flooded electrolytes are generally used for laboratory-based studies. Despite the superior cycling performance achieved for these cells, the metal-anode-excess design deviates from practical applications due to the low anode utilization, highly inflated coulombic efficiency, and undesirable volumetric capacity. In contrast, anode-free configurations can overcome these drawbacks while reducing fabrication costs and improving cell safety. In this review, the significance of anode-free configurations is elaborated, and different types of anode-free cells are introduced, including reported designs and proposed feasible yet unexplored concepts. The optimization strategies for anode-free lithium, sodium, zinc, and aluminum metal batteries are summarized. Most importantly, the remaining challenges for extending the cycle life of anode-free cells are discussed, and the requirements for anode-free cells to reach practical applications are highlighted. This comprehensive review is expected to draw more attention to anode-free configurations and bring new inspiration to the design of high-energy metal batteries.
Graphic Abstract
Anode-free metal batteries can deliver higher energy densities than traditional anode-excess metal batteries and metal-ion batteries. Yet the cycle life of anode-free cells is limited by the non-planar growth and low coulombic efficiency of the metal anodes. In this review, we not only systematically elaborate the working/failure mechanisms and achieved progress for the reported anode-free Li/Na/Zn/Al battery systems, but also propose a series of conceptually-feasible yet unexplored anode-free systems.













Similar content being viewed by others
References
Xu, W., Wang, J.L., Ding, F., et al.: Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 7, 513–537 (2014). https://doi.org/10.1039/c3ee40795k
Cheng, X.B., Zhang, R., Zhao, C.Z., et al.: Toward safe lithium metal anode in rechargeable batteries: a review. Chem. Rev. 117, 10403–10473 (2017). https://doi.org/10.1021/acs.chemrev.7b00115
Whittingham, M.S.: Lithium batteries and cathode materials. Chem. Rev. 104, 4271–4302 (2004). https://doi.org/10.1021/cr020731c
Winter, M., Barnett, B., Xu, K.: Before Li ion batteries. Chem. Rev. 118, 11433–11456 (2018). https://doi.org/10.1021/acs.chemrev.8b00422
Lin, D.C., Liu, Y.Y., Cui, Y.: Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 12, 194–206 (2017). https://doi.org/10.1038/nnano.2017.16
Zhamu, A., Chen, G.R., Liu, C.G., et al.: Reviving rechargeable lithium metal batteries: enabling next-generation high-energy and high-power cells. Energy Environ. Sci. 5, 5701–5707 (2012). https://doi.org/10.1039/c2ee02911a
Guo, Y.P., Li, H.Q., Zhai, T.Y.: Reviving lithium-metal anodes for next-generation high-energy batteries. Adv. Mater. 29, 1700007 (2017). https://doi.org/10.1002/adma.201700007
Liu, J., Bao, Z.N., Cui, Y., et al.: Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 4, 180–186 (2019). https://doi.org/10.1038/s41560-019-0338-x
Kim, M.S., Ryu, J.H., et al.: Langmuir–Blodgett artificial solid-electrolyte interphases for practical lithium metal batteries. Nat. Energy 3, 889–898 (2018). https://doi.org/10.1038/s41560-018-0237-6
Louli, A.J., Genovese, M., Weber, R., et al.: Exploring the impact of mechanical pressure on the performance of anode-free lithium metal cells. J. Electrochem. Soc. 166, A1291–A1299 (2019). https://doi.org/10.1149/2.0091908jes
Genovese, M., Louli, A.J., Weber, R., et al.: Measuring the coulombic efficiency of lithium metal cycling in anode-free lithium metal batteries. J. Electrochem. Soc. 165, A3321–A3325 (2018). https://doi.org/10.1149/2.0641814jes
Xiao, J., Li, Q.Y., Bi, Y.J., et al.: Understanding and applying coulombic efficiency in lithium metal batteries. Nat. Energy 5, 561–568 (2020). https://doi.org/10.1038/s41560-020-0648-z
Nanda, S., Gupta, A., Manthiram, A.: A lithium-sulfur cell based on reversible lithium deposition from a Li2S cathode host onto a hostless-anode substrate. Adv. Energy Mater. 8, 1801556 (2018). https://doi.org/10.1002/aenm.201801556
Brown, Z.L., Jurng, S., Lucht, B.L.: Investigation of the lithium solid electrolyte interphase in vinylene carbonate electrolytes using Cu||LiFePO4 cells. J. Electrochem. Soc. 164, A2186–A2189 (2017). https://doi.org/10.1149/2.0021712jes
Qian, J.F., Adams, B.D., Zheng, J.M., et al.: Anode-free rechargeable lithium metal batteries. Adv. Funct. Mater. 26, 7094–7102 (2016). https://doi.org/10.1002/adfm.201602353
Zhang, S.S., Fan, X.L., Wang, C.S.: A tin-plated copper substrate for efficient cycling of lithium metal in an anode-free rechargeable lithium battery. Electrochim. Acta 258, 1201–1207 (2017). https://doi.org/10.1016/j.electacta.2017.11.175
Qian, J.F., Henderson, W.A., Xu, W., et al.: High rate and stable cycling of lithium metal anode. Nat. Commun. 6, 1–9 (2015). https://doi.org/10.1038/ncomms7362
Pei, A., Zheng, G.Y., Shi, F.F., et al.: Nanoscale nucleation and growth of electrodeposited lithium metal. Nano Lett. 17, 1132–1139 (2017). https://doi.org/10.1021/acs.nanolett.6b04755
Nanda, S., Gupta, A., Manthiram, A.: Anode-free full cells: a pathway to high-energy density lithium-metal batteries. Adv. Energy Mater. 11, 2000804 (2021). https://doi.org/10.1002/aenm.202000804
Zou, P.C., Wang, Y., Chiang, S.W., et al.: Directing lateral growth of lithium dendrites in micro-compartmented anode arrays for safe lithium metal batteries. Nat. Commun. 9, 1–9 (2018). https://doi.org/10.1038/s41467-018-02888-8
Niu, C.J., Pan, H.L., Xu, W., et al.: Self-smoothing anode for achieving high-energy lithium metal batteries under realistic conditions. Nat. Nanotechnol. 14, 594–601 (2019). https://doi.org/10.1038/s41565-019-0427-9
Zou, P.C., Chiang, S.W., Li, J., et al.: Ni@Li2O co-axial nanowire based reticular anode: tuning electric field distribution for homogeneous lithium deposition. Energy Storage Mater. 18, 155–164 (2019). https://doi.org/10.1016/j.ensm.2018.09.020
Li, J., Zou, P.C., Chiang, S.W., et al.: A conductive-dielectric gradient framework for stable lithium metal anode. Energy Storage Mater. 24, 700–706 (2020). https://doi.org/10.1016/j.ensm.2019.06.019
Chen, H., Pei, A., Lin, D.C., et al.: Uniform high ionic conducting lithium sulfide protection layer for stable lithium metal anode. Adv. Energy Mater. 9, 1900858 (2019). https://doi.org/10.1002/aenm.201900858
Yan, C., Cheng, X.B., Tian, Y., et al.: Lithium metal anodes: Dual-layered film protected lithium metal anode to enable dendrite-free lithium deposition. Adv. Mater. 30, 1870181 (2018). https://doi.org/10.1002/adma.201870181
Lang, J.L., Long, Y.Z., Qu, J.L., et al.: One-pot solution coating of high quality LiF layer to stabilize Li metal anode. Energy Storage Mater. 16, 85–90 (2019). https://doi.org/10.1016/j.ensm.2018.04.024
Cha, E., Patel, M.D., Park, J., et al.: 2D MoS2 as an efficient protective layer for lithium metal anodes in high-performance Li-S batteries. Nat. Nanotechnol. 13, 337–344 (2018). https://doi.org/10.1038/s41565-018-0061-y
Liu, W., Guo, R., Zhan, B.X., et al.: Artificial solid electrolyte interphase layer for lithium metal anode in high-energy lithium secondary pouch cells. ACS Appl. Energy Mater. 1, 1674–1679 (2018). https://doi.org/10.1021/acsaem.8b00132
Li, N.W., Shi, Y., Yin, Y.X., et al.: A flexible solid electrolyte interphase layer for long-life lithium metal anodes. Angew. Chem. Int. Ed. 57, 1422 (2018). https://doi.org/10.1002/anie.201713193
Jiao, S.H., Ren, X.D., Cao, R.G., et al.: Stable cycling of high-voltage lithium metal batteries in ether electrolytes. Nat. Energy 3, 739–746 (2018). https://doi.org/10.1038/s41560-018-0199-8
Fan, X.L., Chen, L., Borodin, O., et al.: Non-flammable electrolyte enables Li-metal batteries with aggressive cathode chemistries. Nat. Nanotechnol. 13, 715–722 (2018). https://doi.org/10.1038/s41565-018-0183-2
Dong, T.T., Zhang, J.J., Xu, G.J., et al.: A multifunctional polymer electrolyte enables ultra-long cycle-life in a high-voltage lithium metal battery. Energy Environ. Sci. 11, 1197–1203 (2018). https://doi.org/10.1039/c7ee03365f
Zheng, J.M., Engelhard, M.H., Mei, D.H., et al.: Electrolyte additive enabled fast charging and stable cycling lithium metal batteries. Nat. Energy 2, 1–8 (2017). https://doi.org/10.1038/nenergy.2017.12
Han, X.G., Gong, Y.H., Fu, K., et al.: Negating interfacial impedance in garnet-based solid-state Li metal batteries. Nat. Mater. 16, 572–579 (2017). https://doi.org/10.1038/nmat4821
Basile, A., Bhatt, A.I., O’Mullane, A.P.: Stabilizing lithium metal using ionic liquids for long-lived batteries. Nat. Commun. 7, 11794 (2016). https://doi.org/10.1038/ncomms11794
Xie, Z.K., Wu, Z.J., An, X.W., et al.: Anode-free rechargeable lithium metal batteries: progress and prospects. Energy Storage Mater. 32, 386–401 (2020). https://doi.org/10.1016/j.ensm.2020.07.004
Tian, Y., An, Y.L., Wei, C.L., et al.: Recently advances and perspectives of anode-free rechargeable batteries. Nano Energy 78, 105344 (2020). https://doi.org/10.1016/j.nanoen.2020.105344
Neudecker, B.J., Dudney, N.J., Bates, J.B.: “lithium-free” thin-film battery with in situ plated Li anode. J. Electrochem. Soc. 147, 517 (2000). https://doi.org/10.1149/1.1393226
Beyene, T.T., Bezabh, H.K., Weret, M.A., et al.: Concentrated dual-salt electrolyte to stabilize Li metal and increase cycle life of anode free Li-metal batteries. J. Electrochem. Soc. 166, A1501–A1509 (2019). https://doi.org/10.1149/2.0731908jes
Hagos, T., Thirumalraj, B., Huang, C.J., et al.: Locally concentrated LiPF6 in a carbonate-based electrolyte with fluoroethylene carbonate as a diluent for anode-free lithium metal batteries. ACS Appl. Mater. Interfaces 11, 9955–9963 (2019). https://doi.org/10.1021/acsami.8b21052
Kautz, D.J., Tao, L., Mu, L.Q., et al.: Understanding the critical chemistry to inhibit lithium consumption in lean lithium metal composite anodes. J. Mater. Chem. A 6, 16003–16011 (2018). https://doi.org/10.1039/c8ta01715h
Padhi, A.K., Nanjundaswamy, K.S., Goodenough, J.B.: Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J. Electrochem. Soc. 144, 1188–1194 (1997). https://doi.org/10.1149/1.1837571
Zhang, W.J.: Structure and performance of LiFePO4 cathode materials: a review. J. Power Sources 196, 2962–2970 (2011). https://doi.org/10.1016/j.jpowsour.2010.11.113
Seh, Z.W., Sun, Y.M., Zhang, Q.F., et al.: Designing high-energy lithium-sulfur batteries. Chem. Soc. Rev. 45, 5605–5634 (2016). https://doi.org/10.1039/c5cs00410a
Yang, Y., Zheng, G.Y., Misra, S., et al.: High-capacity micrometer-sized Li2S particles as cathode materials for advanced rechargeable lithium-ion batteries. J. Am. Chem. Soc. 134, 15387–15394 (2012). https://doi.org/10.1021/ja3052206
Tan, G.Q., Xu, R., Xing, Z.Y., et al.: Burning lithium in CS2 for high-performing compact Li2S-graphene nanocapsules for Li-S batteries. Nat. Energy 2, 1–10 (2017). https://doi.org/10.1038/nenergy.2017.90
Yang, Y., McDowell, M.T., Jackson, A., et al.: New nanostructured Li2S/silicon rechargeable battery with high specific energy. Nano Lett. 10, 1486–1491 (2010). https://doi.org/10.1021/nl100504q
Bruce, P.G., Freunberger, S.A., Hardwick, L.J., et al.: Li-O2 and Li-S batteries with high energy storage. Nat. Mater. 11, 19–29 (2012). https://doi.org/10.1038/nmat3191
Suo, L.M., Zhu, Y.J., Han, F.D., et al.: Carbon cage encapsulating nano-cluster Li2S by ionic liquid polymerization and pyrolysis for high performance Li-S batteries. Nano Energy 13, 467–473 (2015). https://doi.org/10.1016/j.nanoen.2015.02.021
Lin, Z., Nan, C.Y., Ye, Y.F., et al.: High-performance lithium/sulfur cells with a bi-functionally immobilized sulfur cathode. Nano Energy 9, 408–416 (2014). https://doi.org/10.1016/j.nanoen.2014.08.003
Guo, J.C., Yang, Z.C., Yu, Y.C., et al.: Lithium-sulfur battery cathode enabled by lithium–nitrile interaction. J. Am. Chem. Soc. 135, 763–767 (2013). https://doi.org/10.1021/ja309435f
Nan, C.Y., Lin, Z., Liao, H.G., et al.: Durable carbon-coated Li2S core–shell spheres for high performance lithium/sulfur cells. J. Am. Chem. Soc. 136, 4659–4663 (2014). https://doi.org/10.1021/ja412943h
Hwa, Y., Zhao, J., Cairns, E.J.: Lithium sulfide (Li2S)/graphene oxide nanospheres with conformal carbon coating as a high-rate, long-life cathode for Li/S cells. Nano Lett. 15, 3479–3486 (2015). https://doi.org/10.1021/acs.nanolett.5b00820
Wu, F.X., Lee, J.T., Fan, F.F., et al.: A hierarchical particle-shell architecture for long-term cycle stability of Li2S cathodes. Adv. Mater. 27, 5579–5586 (2015). https://doi.org/10.1002/adma.201502289
Xu, R., Zhang, X.F., Yu, C., et al.: Paving the way for using Li2S batteries. Chemsuschem 7, 2457–2460 (2014). https://doi.org/10.1002/cssc.201402177
Meini, S., Elazari, R., Rosenman, A., et al.: The use of redox mediators for enhancing utilization of Li2S cathodes for advanced Li-S battery systems. J. Phys. Chem. Lett. 5, 915–918 (2014). https://doi.org/10.1021/jz500222f
Paolella, A., Zhu, W., Marceau, H., et al.: Transient existence of crystalline lithium disulfide Li2S2 in a lithium-sulfur battery. J. Power Sources 325, 641–645 (2016). https://doi.org/10.1016/j.jpowsour.2016.06.086
Fu, Y.Z., Su, Y.S., Manthiram, A.: Highly reversible lithium/dissolved polysulfide batteries with carbon nanotube electrodes. Angew. Chem. Int. Ed. 52, 6930–6935 (2013). https://doi.org/10.1002/anie.201301250
Zu, C.X., Fu, Y.Z., Manthiram, A.: Highly reversible Li/dissolved polysulfide batteries with binder-free carbon nanofiber electrodes. J. Mater. Chem. A 1, 10362 (2013). https://doi.org/10.1039/c3ta11958k
Yao, H.B., Zheng, G.Y., Hsu, P.C., et al.: Improving lithium-sulphur batteries through spatial control of sulphur species deposition on a hybrid electrode surface. Nat. Commun. 5, 1–9 (2014). https://doi.org/10.1038/ncomms4943
Wang, H.T., Zhang, Q.F., Yao, H.B., et al.: High electrochemical selectivity of edge versus terrace sites in two-dimensional layered MoS2 materials. Nano Lett. 14, 7138–7144 (2014). https://doi.org/10.1021/nl503730c
Ogasawara, T., Débart, A., Holzapfel, M., et al.: Rechargeable Li2O2 electrode for lithium batteries. J. Am. Chem. Soc. 128, 1390–1393 (2006). https://doi.org/10.1021/ja056811q
Zhang, S.S., Foster, D., Read, J.: Discharge characteristic of a non-aqueous electrolyte Li/O2 battery. J. Power Sources 195, 1235–1240 (2010). https://doi.org/10.1016/j.jpowsour.2009.08.088
Lu, J., Li, L., Park, J.B., et al.: Aprotic and aqueous Li-O2 batteries. Chem. Rev. 114, 5611–5640 (2014). https://doi.org/10.1021/cr400573b
Lu, J., Lee, Y.J., Luo, X., et al.: A lithium-oxygen battery based on lithium superoxide. Nature 529, 377–382 (2016). https://doi.org/10.1038/nature16484
Lau, K.C., Curtiss, L.A., Greeley, J.: Density functional investigation of the thermodynamic stability of lithium oxide bulk crystalline structures as a function of oxygen pressure. J. Phys. Chem. C 115, 23625–23633 (2011). https://doi.org/10.1021/jp206796h
Sangster, J., Pelton, A.D.: The Li-O (lithium-oxygen) system. J. Phase Equilibria 13, 296–299 (1992). https://doi.org/10.1007/BF02667558
Xu, S.M., Das, S.K., Archer, L.A.: The Li-CO2 battery: a novel method for CO2 capture and utilization. RSC Adv. 3, 6656 (2013). https://doi.org/10.1039/c3ra40394g
Yang, S.X., Qiao, Y., He, P., et al.: A reversible lithium-CO2 battery with Ru nanoparticles as a cathode catalyst. Energy Environ. Sci. 10, 972–978 (2017). https://doi.org/10.1039/c6ee03770d
Zhang, Z., Wang, X.G., Zhang, X., et al.: Verifying the rechargeability of Li-CO2 batteries on working cathodes of Ni nanoparticles highly dispersed on N-doped graphene. Adv. Sci. 5, 1700567 (2018). https://doi.org/10.1002/advs.201700567
Liu, Y.L., Wang, R., Lyu, Y.C., et al.: Rechargeable Li/CO2-O2 (2:1) battery and Li/CO2 battery. Energy Environ. Sci. 7, 677–681 (2014). https://doi.org/10.1039/c3ee43318h
Garcia-Lastra, J.M., Myrdal, J.S.G., Christensen, R., et al.: DFT+U study of polaronic conduction in Li2O2 and Li2CO3: implications for Li-air batteries. J. Phys. Chem. C 117, 5568–5577 (2013). https://doi.org/10.1021/jp3107809
Zhang, Z., Zhang, Q., Chen, Y.N., et al.: The first introduction of graphene to rechargeable Li-CO2 batteries. Angew. Chem. Int. Ed. 54, 6550–6553 (2015). https://doi.org/10.1002/anie.201501214
Liu, B., Sun, Y.L., Liu, L.Y., et al.: Recent advances in understanding Li-CO2 electrochemistry. Energy Environ. Sci. 12, 887–922 (2019). https://doi.org/10.1039/c8ee03417f
Yan, K., Lu, Z.D., Lee, H.W., et al.: Selective deposition and stable encapsulation of lithium through heterogeneous seeded growth. Nat. Energy 1, 1–8 (2016). https://doi.org/10.1038/nenergy.2016.10
Zhang, S.S., Fan, X.L., Wang, C.S.: An in situ enabled lithium metal battery by plating lithium on a copper current collector. Electrochem. Commun. 89, 23–26 (2018). https://doi.org/10.1016/j.elecom.2018.02.011
Li, S., Jiang, M.W., Xie, Y., et al.: Developing high-performance lithium metal anode in liquid electrolytes: challenges and progress. Adv. Mater. 30, 1706375 (2018). https://doi.org/10.1002/adma.201706375
Kang, T., Zhao, J.H., Guo, F., et al.: Dendrite-free lithium anodes enabled by a commonly used copper antirusting agent. ACS Appl. Mater. Interfaces 12, 8168–8175 (2020). https://doi.org/10.1021/acsami.9b19655
Lee, Y.G., Fujiki, S., Jung, C., et al.: High-energy long-cycling all-solid-state lithium metal batteries enabled by silver-carbon composite anodes. Nat. Energy 5, 299–308 (2020). https://doi.org/10.1038/s41560-020-0575-z
Pande, V., Viswanathan, V.: Computational screening of current collectors for enabling anode-free lithium metal batteries. ACS Energy Lett. 4, 2952–2959 (2019). https://doi.org/10.1021/acsenergylett.9b02306
Zhang, R., Li, N.W., Cheng, X.B., et al.: Advanced micro/nanostructures for lithium metal anodes. Adv. Sci. (2017). https://doi.org/10.1002/advs.201770011
Jin, S., Jiang, Y., Ji, H.X., et al.: Advanced 3D current collectors for lithium-based batteries. Adv. Mater. 30, 1802014 (2018). https://doi.org/10.1002/adma.201802014
Zheng, J.X., Kim, M.S., Tu, Z.Y., et al.: Regulating electrodeposition morphology of lithium: Towards commercially relevant secondary Li metal batteries. Chem. Soc. Rev. 49, 2701–2750 (2020). https://doi.org/10.1039/c9cs00883g
Umh, H.N., Park, J., Yeo, J., et al.: Lithium metal anode on a copper dendritic superstructure. Electrochem. Commun. 99, 27–31 (2019). https://doi.org/10.1016/j.elecom.2018.12.015
Liu, H.D., Yue, X.J., Xing, X., et al.: A scalable 3D lithium metal anode. Energy Storage Mater. 16, 505–511 (2019). https://doi.org/10.1016/j.ensm.2018.09.021
Xiang, H.F., Shi, P.C., Bhattacharya, P., et al.: Enhanced charging capability of lithium metal batteries based on lithium bis(trifluoromethanesulfonyl)imide-lithium bis(oxalato)borate dual-salt electrolytes. J. Power Sources 318, 170–177 (2016). https://doi.org/10.1016/j.jpowsour.2016.04.017
Li, Y., Li, Y., Pei, A., et al.: Atomic structure of sensitive battery materials and interfaces revealed by cryo-electron microscopy. Science 358, 506–510 (2017). https://doi.org/10.1126/science.aam6014
Aurbach, D., Zaban, A., Gofer, Y., et al.: Recent studies of the lithium-liquid electrolyte interface electrochemical, morphological and spectral studies of a few important systems. J. Power Sources 54, 76–84 (1995). https://doi.org/10.1016/0378-7753(94)02044-4
Xu, R., Cheng, X.B., Yan, C., et al.: Artificial interphases for highly stable lithium metal anode. Matter 1, 317–344 (2019). https://doi.org/10.1016/j.matt.2019.05.016
Wondimkun, Z.T., Beyene, T.T., Weret, M.A., et al.: Binder-free ultra-thin graphene oxide as an artificial solid electrolyte interphase for anode-free rechargeable lithium metal batteries. J. Power Sources 450, 227589 (2020). https://doi.org/10.1016/j.jpowsour.2019.227589
Tu, Z.Y., Zachman, M.J., Choudhury, S., et al.: Stabilizing protic and aprotic liquid electrolytes at high-bandgap oxide interphases. Chem. Mater. 30, 5655–5662 (2018). https://doi.org/10.1021/acs.chemmater.8b01996
Monroe, C., Newman, J.: The impact of elastic deformation on deposition kinetics at lithium/polymer interfaces. J. Electrochem. Soc. 152, A396 (2005). https://doi.org/10.1149/1.1850854
Ferrese, A., Newman, J.: Mechanical deformation of a lithium-metal anode due to a very stiff separator. J. Electrochem. Soc. 161, A1350–A1359 (2014). https://doi.org/10.1149/2.0911409jes
Lee, C., Wei, X., Kysar, J.W., et al.: Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 321, 385–388 (2008). https://doi.org/10.1126/science.1157996
Assegie, A.A., Chung, C.C., Tsai, M.C., et al.: Multilayer-graphene-stabilized lithium deposition for anode-free lithium-metal batteries. Nanoscale 11, 2710–2720 (2019). https://doi.org/10.1039/c8nr06980h
Assegie, A.A., Cheng, J.H., Kuo, L.M., et al.: Polyethylene oxide film coating enhances lithium cycling efficiency of an anode-free lithium-metal battery. Nanoscale 10, 6125–6138 (2018). https://doi.org/10.1039/c7nr09058g
Abrha, L.H., Zegeye, T.A., Hagos, T.T., et al.: Li7La2.75Ca0.25Zr1.75Nb0.25O12@LiClO4 composite film derived solid electrolyte interphase for anode-free lithium metal battery. Electrochim. Acta 325, 134825 (2019). https://doi.org/10.1016/j.electacta.2019.134825
Ren, X.D., Zou, L.F., Cao, X., et al.: Enabling high-voltage lithium-metal batteries under practical conditions. Joule 3, 1662–1676 (2019). https://doi.org/10.1016/j.joule.2019.05.006
Chao, D.L., Zhu, C.R., Yang, P.H., et al.: Array of nanosheets render ultrafast and high-capacity Na-ion storage by tunable pseudocapacitance. Nat. Commun. 7, 1–8 (2016). https://doi.org/10.1038/ncomms12122
Koch, V.R., Young, J.H.: 2-methyltetrahydrofuran: lithium hexafluoroarsenate: a superior electrolyte for the secondary lithium electrode. Science 204, 499–501 (1979). https://doi.org/10.1126/science.204.4392.499
Abraham, K.M., Goldman, J.L., Natwig, D.L.: Characterization of ether electrolytes for rechargeable lithium cells. J. Electrochem. Soc. 129, 2404–2409 (1982). https://doi.org/10.1149/1.2123556
Xu, K.: Electrolytes and interphases in Li-ion batteries and beyond. Chem. Rev. 114, 11503–11618 (2014). https://doi.org/10.1021/cr500003w
Yu, Z.A., Wang, H.S., Kong, X., et al.: Molecular design for electrolyte solvents enabling energy-dense and long-cycling lithium metal batteries. Nat. Energy 5, 526–533 (2020). https://doi.org/10.1038/s41560-020-0634-5
Jote, B.A., Beyene, T.T., Sahalie, N.A., et al.: Effect of diethyl carbonate solvent with fluorinated solvents as electrolyte system for anode free battery. J. Power Sources 461, 228102 (2020). https://doi.org/10.1016/j.jpowsour.2020.228102
Hagos, T.T., Su, W.N., Huang, C.J., et al.: Developing high-voltage carbonate-ether mixed electrolyte via anode-free cell configuration. J. Power Sources 461, 228053 (2020). https://doi.org/10.1016/j.jpowsour.2020.228053
Rodriguez, R., Loeffler, K.E., Edison, R.A., et al.: Effect of the electrolyte on the cycling efficiency of lithium-limited cells and their morphology studied through in situ optical imaging. ACS Appl. Energy Mater. 1, 5830–5835 (2018). https://doi.org/10.1021/acsaem.8b01194
Hagos, T.M., Berhe, G.B., Hagos, T.T., et al.: Dual electrolyte additives of potassium hexafluorophosphate and tris(trimethylsilyl) phosphite for anode-free lithium metal batteries. Electrochim. Acta 316, 52–59 (2019). https://doi.org/10.1016/j.electacta.2019.05.061
Sahalie, N.A., Assegie, A.A., Su, W.N., et al.: Effect of bifunctional additive potassium nitrate on performance of anode free lithium metal battery in carbonate electrolyte. J. Power Sources 437, 226912 (2019). https://doi.org/10.1016/j.jpowsour.2019.226912
Seo, D.M., Borodin, O., Han, S.D., et al.: Electrolyte solvation and ionic association II. Acetonitrile-lithium salt mixtures: highly dissociated salts. J. Electrochem. Soc. 159, A1489–A1500 (2012). https://doi.org/10.1149/2.035209jes
Beyene, T.T., Jote, B.A., Wondimkun, Z.T., et al.: Effects of concentrated salt and resting protocol on solid electrolyte interface formation for improved cycle stability of anode-free lithium metal batteries. ACS Appl. Mater. Interfaces 11, 31962–31971 (2019). https://doi.org/10.1021/acsami.9b09551
Nilsson, V., Kotronia, A., Lacey, M., et al.: Highly concentrated LiTFSI-EC electrolytes for lithium metal batteries. ACS Appl. Energy Mater. 3, 200–207 (2020). https://doi.org/10.1021/acsaem.9b01203
Alvarado, J., Schroeder, M.A., Pollard, T.P., et al.: Bisalt ether electrolytes: a pathway towards lithium metal batteries with Ni-rich cathodes. Energy Environ. Sci. 12, 780–794 (2019). https://doi.org/10.1039/c8ee02601g
Rodriguez, R., Edison, R.A., Stephens, R.M., et al.: Separator-free and concentrated LiNO3 electrolyte cells enable uniform lithium electrodeposition. J. Mater. Chem. A 8, 3999–4006 (2020). https://doi.org/10.1039/c9ta10929c
Weber, R., Genovese, M., Louli, A.J., et al.: Long cycle life and dendrite-free lithium morphology in anode-free lithium pouch cells enabled by a dual-salt liquid electrolyte. Nat. Energy 4, 683–689 (2019). https://doi.org/10.1038/s41560-019-0428-9
Louli, A.J., Eldesoky, A., Weber, R., et al.: Diagnosing and correcting anode-free cell failure via electrolyte and morphological analysis. Nat. Energy 5, 693–702 (2020)
Genovese, M., Louli, A.J., Weber, R., et al.: Hot formation for improved low temperature cycling of anode-free lithium metal batteries. J. Electrochem. Soc. 166, A3342–A3347 (2019). https://doi.org/10.1149/2.0661914jes
Kim, S., Jung, C., Kim, H., et al.: The role of interlayer chemistry in Li-metal growth through a garnet-type solid electrolyte. Adv. Energy Mater. 10, 1903993 (2020). https://doi.org/10.1002/aenm.201903993
Ye, T.T., Li, L.H., Zhang, Y.: Recent progress in solid electrolytes for energy storage devices. Adv. Funct. Mater. 30, 2000077 (2020). https://doi.org/10.1002/adfm.202000077
Chung, S.H., Manthiram, A.: A Li2S-TiS2-electrolyte composite for stable Li2S-based lithium-sulfur batteries. Adv. Energy Mater. 9, 1901397 (2019). https://doi.org/10.1002/aenm.201901397
Chen, J., Xiang, J.W., Chen, X., et al.: Li2S-based anode-free full batteries with modified Cu current collector. Energy Storage Mater. 30, 179–186 (2020). https://doi.org/10.1016/j.ensm.2020.05.009
Nanda, S., Bhargav, A., Manthiram, A.: Anode-free, lean-electrolyte lithium-sulfur batteries enabled by tellurium-stabilized lithium deposition. Joule 4, 1121–1135 (2020). https://doi.org/10.1016/j.joule.2020.03.020
Cohn, A.P., Muralidharan, N., Carter, R., et al.: Anode-free sodium battery through in situ plating of sodium metal. Nano Lett. 17, 1296–1301 (2017). https://doi.org/10.1021/acs.nanolett.6b05174
Mazzali, F., Orzech, M.W., Adomkevicius, A., et al.: Designing a high-power sodium-ion battery by in situ metal plating. ACS Appl. Energy Mater. 2, 344–353 (2019). https://doi.org/10.1021/acsaem.8b01361
Liu, S., Tang, S., Zhang, X.Y., et al.: Porous Al current collector for dendrite-free Na metal anodes. Nano Lett. 17, 5862–5868 (2017). https://doi.org/10.1021/acs.nanolett.7b03185
Rudola, A., Gajjela, S.R., Balaya, P.: High energy density in situ sodium plated battery with current collector foil as anode. Electrochem. Commun. 86, 157–160 (2018). https://doi.org/10.1016/j.elecom.2017.12.013
Tang, S., Qiu, Z., Wang, X.Y., et al.: A room-temperature sodium metal anode enabled by a sodiophilic layer. Nano Energy 48, 101–106 (2018). https://doi.org/10.1016/j.nanoen.2018.03.039
Cohn, A.P., Metke, T., Donohue, J., et al.: Rethinking sodium-ion anodes as nucleation layers for anode-free batteries. J. Mater. Chem. A 6, 23875–23884 (2018). https://doi.org/10.1039/c8ta05911j
Lee, M.E., Lee, S., Choi, J., et al.: Anode-free sodium metal batteries based on nanohybrid core-shell templates. Small 15, 1901274 (2019). https://doi.org/10.1002/smll.201901274
Tanwar, M., Bezabh, H.K., Basu, S., et al.: Investigation of sodium plating and stripping on a bare current collector with different electrolytes and cycling protocols. ACS Appl. Mater. Interfaces 11, 39746–39756 (2019). https://doi.org/10.1021/acsami.9b10097
Zhou, D., Chen, Y., Li, B.H., et al.: A stable quasi-solid-state sodium-sulfur battery. Angew. Chem. Int. Ed. 57, 10168–10172 (2018). https://doi.org/10.1002/anie.201805008
Wei, S.Y., Xu, S.M., Agrawral, A., et al.: A stable room-temperature sodium-sulfur battery. Nat. Commun. 7, 1–10 (2016). https://doi.org/10.1038/ncomms11722
Wang, Y.-X., Zhang, B., Lai, W., et al.: Room-temperature sodium–sulfur batteries: a comprehensive review on research progress and cell chemistry. Adv. Energy Mater. 7, 1602829 (2017)
Wang, Y.Z., Zhou, D., Palomares, V., et al.: Revitalising sodium–sulfur batteries for non-high-temperature operation: a crucial review. Energy Environ. Sci. 13, 3848–3879 (2020). https://doi.org/10.1039/d0ee02203a
Syali, M.S., Kumar, D., Mishra, K., et al.: Recent advances in electrolytes for room-temperature sodium-sulfur batteries: a review. Energy Storage Mater. 31, 352–372 (2020). https://doi.org/10.1016/j.ensm.2020.06.023
Xu, X.F., Zhou, D., Qin, X.Y., et al.: A room-temperature sodium-sulfur battery with high capacity and stable cycling performance. Nat. Commun. 9, 1–12 (2018). https://doi.org/10.1038/s41467-018-06443-3
Parker, J.F., Chervin, C.N., Pala, I.R., et al.: Rechargeable nickel–3D zinc batteries: an energy-dense, safer alternative to lithium-ion. Science 356, 415–418 (2017). https://doi.org/10.1126/science.aak9991
Blanc, L.E., Kundu, D.P., Nazar, L.F.: Scientific challenges for the implementation of Zn-ion batteries. Joule 4, 771–799 (2020). https://doi.org/10.1016/j.joule.2020.03.002
Zhu, Y.P., Cui, Y., Alshareef, H.N.: An anode-free Zn-MnO2 battery. Nano Lett. 21, 1446–1453 (2021). https://doi.org/10.1021/acs.nanolett.0c04519
Song, M., Tan, H., Chao, D.L., et al.: Recent advances in Zn-ion batteries. Adv. Funct. Mater. 28, 1802564 (2018). https://doi.org/10.1002/adfm.201802564
Zhang, N., Cheng, F.Y., Liu, Y.C., et al.: Cation-deficient spinel ZnMn2O4 cathode in Zn(CF3SO3)2 electrolyte for rechargeable aqueous Zn-ion battery. J. Am. Chem. Soc. 138, 12894–12901 (2016). https://doi.org/10.1021/jacs.6b05958
Sun, W., Wang, F., Zhang, B., et al.: A rechargeable zinc-air battery based on zinc peroxide chemistry. Science 371, 46–51 (2021). https://doi.org/10.1126/science.abb9554
Chao, D.L., Zhou, W.H., Ye, C., et al.: An electrolytic Zn-MnO2 battery for high-voltage and scalable energy storage. Angew. Chem. Int. Ed. 58, 7823–7828 (2019). https://doi.org/10.1002/anie.201904174
Wang, F., Borodin, O., Gao, T., et al.: Highly reversible zinc metal anode for aqueous batteries. Nat. Mater. 17, 543–549 (2018). https://doi.org/10.1038/s41563-018-0063-z
Zhong, C., Liu, B., Ding, J., et al.: Decoupling electrolytes towards stable and high-energy rechargeable aqueous zinc-manganese dioxide batteries. Nat. Energy 5, 440–449 (2020). https://doi.org/10.1038/s41560-020-0584-y
Zeng, Y.X., Zhang, X.Y., Qin, R.F., et al.: Dendrite-free zinc deposition induced by multifunctional CNT frameworks for stable flexible Zn-ion batteries. Adv. Mater. 31, 1903675 (2019). https://doi.org/10.1002/adma.201903675
Zheng, J.X., Zhao, Q., Tang, T., et al.: Reversible epitaxial electrodeposition of metals in battery anodes. Science 366, 645–648 (2019). https://doi.org/10.1126/science.aax6873
Deng, C.B., Xie, X.S., Han, J.W., et al.: A sieve-functional and uniform-porous Kaolin layer toward stable zinc metal anode. Adv. Funct. Mater. 30, 2000599 (2020). https://doi.org/10.1002/adfm.202000599
Zhao, Z.M., Zhao, J.W., Hu, Z.L., et al.: Long-life and deeply rechargeable aqueous Zn anodes enabled by a multifunctional brightener-inspired interphase. Energy Environ. Sci. 12, 1938–1949 (2019). https://doi.org/10.1039/c9ee00596j
Tu, J.G., Song, W.L., Lei, H.P., et al.: Nonaqueous rechargeable aluminum batteries: progresses, challenges, and perspectives. Chem. Rev. 121, 4903–4961 (2021). https://doi.org/10.1021/acs.chemrev.0c01257
Zafar, Z.A., Imtiaz, S., Razaq, R., et al.: Cathode materials for rechargeable aluminum batteries: current status and progress. J. Mater. Chem. A 5, 5646–5660 (2017). https://doi.org/10.1039/c7ta00282c
Zhao, Q., Zheng, J.X., Deng, Y., et al.: Regulating the growth of aluminum electrodeposits: towards anode-free Al batteries. J. Mater. Chem. A 8, 23231–23238 (2020). https://doi.org/10.1039/d0ta08505g
Albertus, P., Babinec, S., Litzelman, S., et al.: Status and challenges in enabling the lithium metal electrode for high-energy and low-cost rechargeable batteries. Nat. Energy 3, 16–21 (2018). https://doi.org/10.1038/s41560-017-0047-2
Liu, Y.Y., Xiong, S.Z., Wang, J.L., et al.: Dendrite-free lithium metal anode enabled by separator engineering via uniform loading of lithiophilic nucleation sites. Energy Storage Mater. 19, 24–30 (2019). https://doi.org/10.1016/j.ensm.2018.10.015
Shin, W.K., Kannan, A.G., Kim, D.W.: Effective suppression of dendritic lithium growth using an ultrathin coating of nitrogen and sulfur codoped graphene nanosheets on polymer separator for lithium metal batteries. ACS Appl. Mater. Interfaces 7, 23700–23707 (2015). https://doi.org/10.1021/acsami.5b07730
Ryou, M.H., Lee, D.J., Lee, J.N., et al.: Excellent cycle life of lithium-metal anodes in lithium-ion batteries with mussel-inspired polydopamine-coated separators. Adv. Energy Mater. 2, 645–650 (2012). https://doi.org/10.1002/aenm.201100687
Liu, W., Mi, Y.Y., Weng, Z., et al.: Functional metal–organic framework boosting lithium metal anode performance via chemical interactions. Chem. Sci. 8, 4285–4291 (2017). https://doi.org/10.1039/c7sc00668c
Jin, R., Fu, L.X., Zhou, H.L., et al.: High Li+ ionic flux separator enhancing cycling stability of lithium metal anode. ACS Sustainable Chem. Eng. 6, 2961–2968 (2018). https://doi.org/10.1021/acssuschemeng.7b02502
Zhao, C.Z., Chen, P.Y., Zhang, R., et al.: An ion redistributor for dendrite-free lithium metal anodes. Sci. Adv. 4, eaat3446 (2018). https://doi.org/10.1126/sciadv.aat3446
Wang, Y.N., Shi, L.Y., Zhou, H.L., et al.: Polyethylene separators modified by ultrathin hybrid films enhancing lithium ion transport performance and Li-metal anode stability. Electrochim. Acta 259, 386–394 (2018). https://doi.org/10.1016/j.electacta.2017.10.120
Hao, X., Zhu, J., Jiang, X., et al.: Ultrastrong polyoxyzole nanofiber membranes for dendrite-proof and heat-resistant battery separators. Nano Lett. 16, 2981–2987 (2016)
Tian, H.Z., Seh, Z.W., Yan, K., et al.: Theoretical investigation of 2D layered materials as protective films for lithium and sodium metal anodes. Adv. Energy Mater. 7, 1602528 (2017). https://doi.org/10.1002/aenm.201602528
Wang, D., Qin, C.C., Li, X.L., et al.: Synchronous healing of Li metal anode via asymmetrical bidirectional current. Science 23, 100781 (2020). https://doi.org/10.1016/j.isci.2019.100781
Lu, D.P., Shao, Y.Y., Lozano, T., et al.: Failure mechanism for fast-charged lithium metal batteries with liquid electrolytes. Adv. Energy Mater. 5, 1400993 (2015). https://doi.org/10.1002/aenm.201400993
Yan, K., Wang, J.Y., Zhao, S.Q., et al.: Temperature-dependent nucleation and growth of dendrite-free lithium metal anodes. Angew. Chem. Int. Ed. 58, 11364–11368 (2019). https://doi.org/10.1002/anie.201905251
Li, L., Basu, S., Wang, Y.P., et al.: Self-heating-induced healing of lithium dendrites. Science 359, 1513–1516 (2018). https://doi.org/10.1126/science.aap8787
Hundekar, P., Basu, S., Fan, X.L., et al.: In situ healing of dendrites in a potassium metal battery. Proc. Natl. Acad. Sci. USA 117, 5588–5594 (2020). https://doi.org/10.1073/pnas.1915470117
Yang, Q., Liang, G.J., Guo, Y., et al.: Do zinc dendrites exist in neutral zinc batteries: a developed electrohealing strategy to in situ rescue in-service batteries. Adv. Mater. 31, 1903778 (2019). https://doi.org/10.1002/adma.201903778
Yang, H., Fey, E.O., Trimm, B.D., et al.: Effects of pulse plating on lithium electrodeposition, morphology and cycling efficiency. J. Power Sources 272, 900–908 (2014). https://doi.org/10.1016/j.jpowsour.2014.09.026
Chen, J., Li, Q., Pollard, T.P., et al.: Electrolyte design for Li metal-free Li batteries. Mater. Today 39, 118–126 (2020)
Wang, X.F., Zhang, M.H., Alvarado, J., et al.: New insights on the structure of electrochemically deposited lithium metal and its solid electrolyte interphases via cryogenic TEM. Nano Lett. 17, 7606–7612 (2017). https://doi.org/10.1021/acs.nanolett.7b03606
Huang, W., Attia, P.M., Wang, H.S., et al.: Evolution of the solid–electrolyte interphase on carbonaceous anodes visualized by atomic-resolution cryogenic electron microscopy. Nano Lett. 19, 5140–5148 (2019). https://doi.org/10.1021/acs.nanolett.9b01515
Wang, J.Y., Huang, W., Pei, A., et al.: Improving cyclability of Li metal batteries at elevated temperatures and its origin revealed by cryo-electron microscopy. Nat. Energy 4, 664–670 (2019). https://doi.org/10.1038/s41560-019-0413-3
Fang, C., Li, J., Zhang, M., et al.: Quantifying inactive lithium in lithium metal batteries. Nature 572, 511–515 (2019). https://doi.org/10.1038/s41586-019-1481-z
Fang, C.C., Wang, X.F., Meng, Y.S.: Key issues hindering a practical lithium-metal anode. Trends Chem. 1, 152–158 (2019). https://doi.org/10.1016/j.trechm.2019.02.015
Aryanfar, A., Brooks, D.J., Colussi, A.J., et al.: Quantifying the dependence of dead lithium losses on the cycling period in lithium metal batteries. Phys. Chem. Chem. Phys. 16, 24965–24970 (2014). https://doi.org/10.1039/c4cp03590a
Chen, K.H., Wood, K.N., Kazyak, E., et al.: Dead lithium: mass transport effects on voltage, capacity, and failure of lithium metal anodes. J. Mater. Chem. A 5, 11671–11681 (2017). https://doi.org/10.1039/c7ta00371d
Zou, P.C., Chiang, S.W., Zhan, H.C., et al.: A periodic “self-correction” scheme for synchronizing lithium plating/stripping at ultrahigh cycling capacity. Adv. Funct. Mater. 30, 1910532 (2020). https://doi.org/10.1002/adfm.201910532
Tu, Z.Y., Choudhury, S., Zachman, M.J., et al.: Fast ion transport at solid-solid interfaces in hybrid battery anodes. Nat. Energy 3, 310–316 (2018). https://doi.org/10.1038/s41560-018-0096-1
Sun, F., Zhou, D., He, X., et al.: Morphological reversibility of modified Li-based anodes for next-generation batteries. ACS Energy Lett. 5, 152–161 (2020)
Harry, K.J., Hallinan, D.T., Parkinson, D.Y., et al.: Detection of subsurface structures underneath dendrites formed on cycled lithium metal electrodes. Nat. Mater. 13, 69–73 (2014). https://doi.org/10.1038/nmat3793
Yan, Y.Y., Cheng, C., Zhang, L., et al.: Lithium–sulfur batteries: deciphering the reaction mechanism of lithium–sulfur batteries by in situ/operando synchrotron-based characterization techniques. Adv. Energy Mater. 9, 1970062 (2019). https://doi.org/10.1002/aenm.201970062
Wang, J.J., Eng, C., Chen-Wiegart, Y.C.K., et al.: Probing three-dimensional sodiation-desodiation equilibrium in sodium-ion batteries by in situ hard X-ray nanotomography. Nat. Commun. 6, 1–9 (2015). https://doi.org/10.1038/ncomms8496
Cao, Y.L., Li, M., Lu, J., et al.: Bridging the academic and industrial metrics for next-generation practical batteries. Nat. Nanotechnol. 14, 200–207 (2019). https://doi.org/10.1038/s41565-019-0371-8
Chen, S.R., Niu, C.J., Lee, H., et al.: Critical parameters for evaluating coin cells and pouch cells of rechargeable Li-metal batteries. Joule 3, 1094–1105 (2019). https://doi.org/10.1016/j.joule.2019.02.004
Vitins, G., Raekelboom, E.A., Weller, M.T., et al.: Li2CuO2 as an additive for capacity enhancement of lithium ion cells. J. Power Sources 119, 938–942 (2003). https://doi.org/10.1016/S0378-7753(03)00236-2
Jeżowski, P., Fic, K., Crosnier, O., et al.: Lithium rhenium(vii) oxide as a novel material for graphite pre-lithiation in high performance lithium-ion capacitors. J. Mater. Chem. A 4, 12609–12615 (2016). https://doi.org/10.1039/c6ta03810g
Lim, Y.G., Kim, D., Lim, J.M., et al.: Anti-fluorite Li6CoO4 as an alternative lithium source for lithium ion capacitors: an experimental and first principles study. J. Mater. Chem. A 3, 12377–12385 (2015). https://doi.org/10.1039/c5ta00297d
Park, M.S., Lim, Y.G., Park, J.W., et al.: Li2RuO3 as an additive for high-energy lithium-ion capacitors. J. Phys. Chem. C 117, 11471–11478 (2013). https://doi.org/10.1021/jp4005828
Park, M.S., Lim, Y.G., Kim, J.H., et al.: A novel lithium-doping approach for an advanced lithium ion capacitor. Adv. Energy Mater. 1, 1002–1006 (2011). https://doi.org/10.1002/aenm.201100270
Abouimrane, A., Cui, Y.J., Chen, Z.H., et al.: Enabling high energy density Li-ion batteries through Li2O activation. Nano Energy 27, 196–201 (2016). https://doi.org/10.1016/j.nanoen.2016.06.050
Zhang, S.S.: A cost-effective approach for practically viable Li-ion capacitors by using Li2S as an in situ Li-ion source material. J. Mater. Chem. A 5, 14286–14293 (2017). https://doi.org/10.1039/c7ta03923a
Acknowledgements
The authors would like to thank the Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (2017BT01N111), the National Nature Science Foundation of China (Project Nos. 52061160482), Shenzhen Geim Graphene Center, Guangdong Province Science and Technology Department (Project No. 2020A0505100014), Shenzhen Government (Project Nos. JSGG20191129110201725, JCYJ20170412171720306 and JSGG20170414143635496) and Tsinghua Shenzhen International Graduate School Overseas Collaboration Project for financial supports.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Rights and permissions
About this article
Cite this article
Yao, W., Zou, P., Wang, M. et al. Design Principle, Optimization Strategies, and Future Perspectives of Anode-Free Configurations for High-Energy Rechargeable Metal Batteries. Electrochem. Energ. Rev. 4, 601–631 (2021). https://doi.org/10.1007/s41918-021-00106-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41918-021-00106-6