Abstract
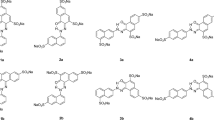
Ionizing radiation is a promising method for dye degradation or textile coloration using commercial azo dyes and small molecular weight organic dyes. Thus, the stability of the molecular structure of an azo dye is important under ionizing radiation. Disperse Blue 79, as an example azo dyes, was irradiated with gamma rays or electron beam (EB) to investigate the radiation-induced effects on the molecular structure. Ultraviolet visible spectroscopy (UV–Vis), nuclear magnetic resonance (NMR) spectra analysis, and mass spectrometry (MS) studies indicated that acetoxy and methoxyl were easily cleaved on the irradiation of the aqueous dye solution but retained a stable structure on the irradiation of the powder form. Gamma rays and EB showed similar effects on the decomposition process. Chromaticity changes using the Lab* method showed that the dye turned to dark yellow and the value of b* of the irradiated dyes increased with the increasing absorbed dose, which indicated that Disperse Blue 79 could be partly decomposed in an aqueous solution with an absorbed dose of 10 kGy. Furthermore, the results demonstrated that the chemical stability of the Disperse Blue 79 under ionizing radiation are different in its powder form with the dye in the aqueous solution.








Similar content being viewed by others
References
E.J. Weber, R.L. Adams, Chemical and sediment-mediated reduction of the Azo dye disperse blue 79. Environ. Sci. Technol. 29, 1163–1170 (1995). https://doi.org/10.1021/es00005a005
F. Li, L. Lv, X. Wang et al., Constructhttps://doi.org/10.1021/acssuschemeng.8b03976ing of dyes suitable for eco-friendly dyeing wool fibers in supercritical carbon dioxide. ACS Sustain. Chem. Eng. 6, 16726–16733 (2018).
A. Aboelnaga, S. Shaarawy, A.G. Hassabo, Polyaconitic acid/functional amine/azo dye composite as a novel hyper-branched polymer for cotton fabric functionalization. Colloids Surfaces B. Biointerfaces. 172, 545–554 (2018). https://doi.org/10.1016/j.colsurfb.2018.09.012
J.-H. Park, U.-Y. Kim, B.-M. Kim et al., Molecular design strategy toward robust organic dyes in thin-film photoanodes. ACS Appl. Energy Mater. 2, 4674–4682 (2019). https://doi.org/10.1021/acsaem.8b02100
A. Villela, M.S.A. van Vuuren, H.M. Willemen et al., Photo-stability of a flavonoid dye in presence of aluminium ions. Dyes Pigments. 162, 222–231 (2019). https://doi.org/10.1016/j.dyepig.2018.10.021
P. Huang, D. Xia, A. Kazlauciunas et al., Dye-mediated interactions in chitosan-based polyelectrolyte/organoclay hybrids for enhanced adsorption of industrial dyes. ACS Appl. Mater. Interface. 11, 11961–11969 (2019). https://doi.org/10.1021/acsami.9b01648
Z. Liu, L. Zhang, F. Dong et al., Preparation of ultrasmall goethite nanorods and their application as heterogeneous fenton reaction catalysts in the degradation of Azo dyes. ACS Appl. Nano Mater. 1, 4170–4178 (2018). https://doi.org/10.1021/acsanm.8b00930
M. Vall, M. Strømme, O. Cheung, Amine-modified mesoporous magnesium carbonate as an effective adsorbent for Azo dyes. ACS Omega. 4, 2973–2979 (2019). https://doi.org/10.1021/acsomega.8b03493
M. Chethana, L.G. Sorokhaibam, V.M. Bhandari et al., Green approach to dye wastewater treatment using biocoagulants. ACS Sustain. Chem. Eng. 4, 2495–2507 (2016). https://doi.org/10.1021/acssuschemeng.5b01553
S.L. Shinde, K.K. Nanda, Photon-free degradation of dyes by Ge/GeO2 porous microstructures. ACS Sustain. Chem. Eng. 7, 6611–6618 (2019). https://doi.org/10.1021/acssuschemeng.8b05549
S.K. Sen, S. Raut, P. Bandyopadhyay et al., Fungal decolouration and degradation of azo dyes: a review. Fungal Biol. Rev. 30, 112–133 (2016). https://doi.org/10.1016/j.fbr.2016.06.003
S. Martínez-López, C. Lucas-Abellán, A. Serrano-Martínez et al., Pulsed light for a cleaner dyeing industry: Azo dye degradation by an advanced oxidation process driven by pulsed light. J. Clean. Prod. 217, 757–766 (2019). https://doi.org/10.1016/j.jclepro.2019.01.230
I.I. Raffainer, P. Rudolf von Rohr, Promoted wet oxidation of the Azo dye orange II under mild conditions. Ind. Eng. Chem. Res. 40, 1083–1089 (2001). https://doi.org/10.1021/ie000629a
L. Szpyrkowicz, C. Juzzolino, S.N. Kaul, S. Daniele et al., Electrochemical oxidation of dyeing baths bearing disperse dyes. Ind. Eng. Chem. Res. 39, 3241–3248 (2000). https://doi.org/10.1021/ie9908480
H. Wang, W. Zhang, J. Zhao et al., Rapid decolorization of phenolic Azo dyes by immobilized laccase with Fe3O4/SiO2 nanoparticles as support. Ind. Eng. Chem. Res. 52, 4401–4407 (2013). https://doi.org/10.1021/ie302627c
L. Szpyrkowicz, R. Cherbanski, G.H. Kelsall, Hydrodynamic effects on the performance of an electrochemical reactor for destruction of disperse dyes. Ind. Eng. Chem. Res. 44, 2058–2068 (2005). https://doi.org/10.1021/ie049444k
X. Li, M. Ma, W. Shao et al., Molecular cloning and functional analysis of a UV-B photoreceptor gene, BpUVR8 (UV Resistance Locus 8), from birch and its role in ABA response. Plant Sci. 274, 294–308 (2018). https://doi.org/10.1016/j.plantsci.2018.06.006
X.X. Feng, L.L. Zhang, J.Y. Chen et al., New insights into solar UV-protective properties of natural dye. J. Clean. Prod. 15, 366–372 (2007). https://doi.org/10.1016/j.jclepro.2005.11.003
R. Miraftab, B. Ramezanzadeh, G. Bahlakeh, An advanced approach for fabricating a reduced graphene oxide-AZO dye/polyurethane composite with enhanced ultraviolet (UV) shielding properties Experimental and first-principles QM modeling. Chem. Eng. J. 321, 159–174 (2017). https://doi.org/10.1016/j.cej.2017.03.124
I. Shahidul, F. Mohammad, High-energy radiation induced sustainable coloration and functional finishing of textile materials. Ind. Eng. Chem. Res. 54, 3727–3745 (2015). https://doi.org/10.1021/acs.iecr.5b00524
M. Yu, W. Li, Z. Wang et al., Covalent immobilization of metal-organic frameworks onto the surface of nylon-a new approach to the functionalization and coloration of textiles. Sci Rep. 6, 22796 (2016). https://doi.org/10.1038/srep22796
N.M. Mallikarjuna, J. Keshavayya, Synthesis, spectroscopic characterization and pharmacological studies on novel sulfamethaxazole based azo dyes. J. King Saud Univ. Sci. 32, 251–259 (2018). https://doi.org/10.1016/j.jksus.2018.04.033
X. Ding, M. Yu, Z. Wang et al., A promising clean way to textile colouration: cotton fabric covalently-bonded with carbon black, cobalt blue, cobalt green, and iron oxide red nanoparticles. Green Chem. 21, 6611–6621 (2019). https://doi.org/10.1039/C9GC02084E
X. Xu, X.J. Ding, J.X. Ao et al., Preparation of amidoxime-based PE/PP fibers for extraction of uranium from aqueous solution. Nucl. Sci. Tech. 30, 20 (2019). https://doi.org/10.1007/s41365-019-0543-0
R.G. Saratale, G.D. Saratale, J.S. Chang et al., Bacterial decolorization and degradation of azo dyes: a review. J. Taiwan Inst. Chem. Eng. 42, 138–157 (2011). https://doi.org/10.1016/j.jtice.2010.06.006
T. Suzuki, S. Timofei, L. Kunrunczi et al., Correlation of aerobic biodegradability of sulfonated azo dyes with the chemical structure. Chemosphere 45, 1–9 (2001). https://doi.org/10.1016/s0045-6535(01)00074-1
S.J. Porobić, A.D. Krstić, D.J. Jovanović et al., Synthesis and thermal properties of arylazo pyridone dyes. Dyes Pigments. 170, 107602 (2019). https://doi.org/10.1016/j.dyepig.2019.107602
Z. Kiayi, T.B. Lotfabad, A. Heidarinasab et al., Microbial degradation of azo dye carmoisine in aqueous medium using Saccharomyces cerevisiae ATCC 9763. J. Hazard. Mater. 373, 608–619 (2019). https://doi.org/10.1016/j.jhazmat.2019.03.111
Y. Mu, K. Rabaey, R.A. Rozendal et al., Decolorization of Azo dyes in bioelectrochemical systems. Environ. Sci. Technol. 43, 5137–5143 (2009). https://doi.org/10.1021/es900057f
J. Qiu, J. Xiao, B. Tang et al., Facile synthesis of novel disperse azo dyes with aromatic hydroxyl group. Dyes Pigments. 160, 524–529 (2019). https://doi.org/10.1016/j.dyepig.2018.08.052
A.D. Broadbent, Y. Mir, M. Lhachimi et al., Continuous dyeing of cotton/polyester and polyester fabrics with reactive and disperse dyes using infrared heat. Ind. Eng. Chem. Res. 46, 2710–2714 (2007). https://doi.org/10.1021/ie0700617
A. Hou, J. Dai, The crystal morphology of C.I. Disperse Blue 79 in supercritical carbon dioxide. Dyes Pigments. 82, 71–75 (2009). https://doi.org/10.1016/j.dyepig.2008.11.004
H. Wang, L. Li, J. Guan et al., Investigation on molecular structures of electron-beam-irradiated low-density polyethylene by rheology measurements. Ind. Eng. Chem. Res. 57, 4298–4310 (2013). https://doi.org/10.1021/acs.iecr.8b00062
R.R. Mather, Aggregate structures of samples of the disperse dye, C.I. Disperse Blue 79. Colloids Surf 37, 131–140 (1989). https://doi.org/10.1016/0166-6622(89)80112-x
K.-M. Park, I. Yoon, S. Lee et al., X-ray crystal structure of C.I. Disperse Blue 79. Dyes Pigments 54, 155–161 (2002). https://doi.org/10.1016/s0143-7208(02)00037-2
M. Wang, R. Yang, W. Wang et al., Radiation-induced decomposition and decoloration of reactive dyes in the presence of H2O2. Radiat. Phys. Chem. 75, 286–291 (2006). https://doi.org/10.1016/j.radphyschem.2005.08.012
J.T. Spadaro, L. Isabelle, V. Renganathan, Hydroxyl radical mediated degradation of Azo dyes evidence for benzene generation. Environ. Sci. Technol. 28, 1389–1393 (1994). https://doi.org/10.1021/es00056a031
J.E. Silveira, A.L. Garcia-Costa, T.O. Cardoso et al., Indirect decolorization of azo dye Disperse Blue 3 by electro-activated persulfate. Electrochim. Acta. 258, 927–932 (2017). https://doi.org/10.1016/j.electacta.2017.11.143
J. Wu, W. Wen, Catalyzed degradation of Azo dyes under ambient conditions. Environ. Sci. Technol. 44, 9123–9127 (2010). https://doi.org/10.1021/es1027234
A. Jabbar, A. Ambreen, S. Riaz et al., A series of new acid dyes; study of solvatochromism, spectroscopy and their application on wool fabric. J. Mol. Struct. 1195, 161–167 (2019). https://doi.org/10.1016/j.molstruc.2019.05.019
L.C. Abbott, S.N. Batchelor, J.N. Moore, Structure and reactivity of thiazolium azo dyes: UV–visible, resonance Raman, NMR, and computational studies of the reaction mechanism in alkaline solution. J. Phys. Chem. A 117, 1853–1871 (2013). https://doi.org/10.1021/jp309536h
C.C. Hsueh, B.Y. Chen, Exploring effects of chemical structure on azo dye decolorization characteristics by Pseudomonas luteola. J. Hazard. Mater. 154, 703–710 (2008). https://doi.org/10.1016/j.jhazmat.2007.10.083
A.S. Ozen, V. Aviyente, Modeling the substituent effect on the oxidative degradation of Azo dyes. J. Phys. Chem. A. 108, 5990–6000 (2004). https://doi.org/10.1021/jp037138z
E. Guerra, F. Gosetti, E. Marengo et al., Study of photostability of three synthetic dyes commonly used in mouthwashes. Microchem. J. 146, 776–781 (2019). https://doi.org/10.1016/j.microc.2019.02.002
H.T. Wang, H.Q. Jiang, R.F. Shen et al., Electron-beam radiation effects on the structure and properties of polypropylene at low dose rates. Nucl. Sci. Tech. 29, 87 (2018). https://doi.org/10.1007/s41365-018-0424-y
H.R. Tan, Z. Xing, W.H. Liu et al., Oxidation effects of poly (ether-ether-ketone) induced by electron-beam irradiation. J. Radiat. Res. Radiat. Process. 37, 020201 (2019) (in Chinese). https://doi.org/10.11889/j.1000-3436.2019.rrj.37.020201
A.Y.L. Tang, C.H. Lee, Y.M. Wang et al., Reverse micellar dyeing of cotton fiber with reactive dyes: A study of the effect of water pH and hardness. ACS Omega. 4, 11808–11814 (2019). https://doi.org/10.1021/acsomega.9b00597
W. Zheng, J. Zou, Synthesis and characterization of blue TiO2/CoAl2O4 complex pigments with good colour and enhanced near-infrared reflectance properties. RSC Adv. 5, 87932–87939 (2015). https://doi.org/10.1039/c5ra17418j
Y.Y. Xie, Z.L. Chen, Z.L. Li et al, Effect of electron-beam irradiation on colority of printing-dyeing wastewater. J. Radiat. Res. Radiat. Process. 36, 060401 (2018) (in Chinese). https://doi.org/10.11889/j.1000-3436.2018.rrj.36.060401.
Acknowledgements
The authors thank Zhejiang Greenland Textile Technology Co., Ltd. for the kind supply of Commercial Disperse Blue 79.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was financially supported by the National Natural Science Foundation of China (Nos. 11875313, 11605274, and 11575277).
Rights and permissions
About this article
Cite this article
Ding, XJ., Yu, M., Zheng, X. et al. Stability study of Disperse Blue 79 under ionizing radiation. NUCL SCI TECH 31, 21 (2020). https://doi.org/10.1007/s41365-020-0724-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41365-020-0724-x