Abstract
Background
Sleep deprivation (SD) impairs pre-stimulus inhibition, but the effect of quetiapine (QET) remains largely unknown.
Objective
This study aimed to investigate the behavioral and cognitive effects of QET in both naïve and sleep-deprived rats.
Materials and methods
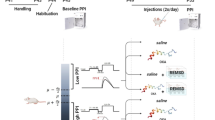
Seven groups (n = 49) of male Wistar Albino rats were used in this study. SD was performed using the modified multiple platform technique in a water tank for 72 h. Our study consists of two experiments investigating the effect of QET on pre-pulse inhibition (PPI) of the acoustic startle reflex. The first experiment tested the effect of short- and long-term administration of QET on PPI response in non-sleeping (NSD) rats. The second experiment used 72 h REM sleep deprivation as a model for SD-induced impairment of the PPI response. Here, we tested the effect of QET on the % PPI of SD rats by short- and long-term intraperitoneal injection at the last 90 min of sleep SD and immediately subsequently tested for PPI.
Results
72 h SD impaired PPI, reduced startle amplitude, and attenuated the PPI% at + 4 dB, + 8 dB, and + 16 dB prepulse intensities. 10 mg/kg short and long-term QET administration completely improved sensorimotor gating deficit, increased startle amplitude, and restored the impaired PPI% at + 4 dB, + 8 dB, and + 16 dB after 72 h SD in rats.
Conclusion
Our results showed short- and long-term administration of QET improved sensorimotor gating deficit in 72 h SD. Further research is required for the etiology of insomnia and the dose-related behavioral effects of QET.




Similar content being viewed by others
References
Sprecher KE, Ferrarelli F, Benca RM. Sleep and plasticity in schizophrenia. Curr Top Behav Neurosci. 2015;25:433–58. https://doi.org/10.1007/7854_2014_366.
Waters F, Chiu V, Atkinson A, Blom JD. Severe sleep deprivation causes hallucinations and a gradual progression toward psychosis with increasing time awake. Front Psychiatry. 2018;10(9):303. https://doi.org/10.3389/fpsyt.2018.00303.
Davies G, Haddock G, Yung AR, Mulligan LD, Kyle SD. A systematic review of the nature and correlates of sleep disturbance in early psychosis. Sleep Med Rev. 2017;31:25–38. https://doi.org/10.1016/j.smrv.2016.01.001.
Malik V, Parthasarathy S. Sleep in intensive care units. Curr Respir Care Reports. 2014;3(2):35–41. https://doi.org/10.1007/s13665-014-0077-1.
Owens J, Gruber R, Brown T, Corkum P, Cortese S, O’Brien L, Stein M, Weiss M. Future research directions in sleep and ADHD: report of a consensus working group. J Atten Disord. 2013;17(7):550–64. https://doi.org/10.1177/1087054712457992.
Pisani MA, Friese RS, Gehlbach BK, Schwab RJ, Weinhouse GL, Jones SF. Sleep in the intensive care unit. Am J Respir Crit Care Med. 2015;191(7):731–8. https://doi.org/10.1164/rccm.201411-2099CI.
Brisch R, Saniotis A, Wolf R, Bielau H, Bernstein HG, Steiner J, Bogerts B, Braun K, Jankowski Z, Kumaratilake J, Henneberg M, Gos T. The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: old fashioned, but still in vogue. Front Psychiatry. 2014;19(5):47. https://doi.org/10.3389/fpsyt.2014.00047.
Kim SA. 5-HT1A and 5-HT2A signaling, desensitization, and downregulation: serotonergic dysfunction and abnormal receptor density in schizophrenia and the prodrome. Cureus. 2021;13(6): e15811. https://doi.org/10.7759/cureus.15811.
Elmenhorst D, Kroll T, Matusch A, Bauer A. Sleep deprivation increases cerebral serotonin 2A receptor binding in humans. Sleep. 2012;35(12):1615–23. https://doi.org/10.5665/sleep.2230.
Herrero JL, Roberts MJ, Delicato LS, Gieselmann MA, Dayan P, Thiele A. Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature. 2008;454(7208):1110–4. https://doi.org/10.1038/nature07141.
Zant JC, Leenaars CHC, Kostin A, Van Someren EJW, Porkka-Heiskanen T. Increases in extracellular serotonin and dopamine metabolite levels in the basal forebrain during sleep deprivation. Brain Res. 2011;1399:40–8. https://doi.org/10.1016/j.brainres.2011.05.008.
Wang X, Wang Z, Cao J, Dong Y, Chen Y. Melatonin alleviates acute sleep deprivation-induced memory loss in mice by suppressing hippocampal ferroptosis. Front Pharmacol. 2021;16(12): 708645. https://doi.org/10.3389/fphar.2021.708645.
Benedict C, Brooks SJ, O’Daly OG, Almèn MS, Morell A, Åberg K, Gingnell M, Schultes B, Hallschmid M, Broman JE, Larsson EM, Schiöth HB. Acute sleep deprivation enhances the brain’s response to hedonic food stimuli: an fMRI study. J Clin Endocrinol Metab. 2012;97(3):E443–7. https://doi.org/10.1210/jc.2011-2759.
Eggers AE. A serotonin hypothesis of schizophrenia. Med Hypotheses. 2013;80(6):791–4. https://doi.org/10.1016/j.mehy.2013.03.013.
Sumiyoshi T, Kunugi H, Nakagome K. Serotonin and dopamine receptors in motivational and cognitive disturbances of schizophrenia. Front Neurosci. 2014;4(8):395. https://doi.org/10.3389/fnins.2014.00395.
World Health Organization. International classification of diseases 10th revision (ICD-10). Geneva: World Health Organization; 1994.
American Psychiatric Association (APA). Diagnostic and statistical manual of mental disorder. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
Iqbal Y, Connell C, Worthington M, Elrafei H, Mulvaney CA, Kaewchaluay C. Quetiapine dose for people with schizophrenia. Cochrane Database Syst Rev. 2019;2019(7):CD013372. https://doi.org/10.1002/14651858.CD013372.
KivircikAkdede BB, Alptekin K, Kitiş A, Arkar H, Akvardar Y. Effects of quetiapine on cognitive functions in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(2):233–8. https://doi.org/10.1016/j.pnpbp.2004.11.005.
Li P, Snyder GL, Vanover KE. Dopamine targeting drugs for the treatment of schizophrenia: past, present and future. Curr Top Med Chem. 2016;16(29):3385–403. https://doi.org/10.2174/1568026616666160608084834.
Björkholm C, Jardemark K, Marcus MM, Malmerfelt A, Nyberg S, Schilström B, Svensson TH. Role of concomitant inhibition of the norepinephrine transporter for the antipsychotic effect of quetiapine. Eur Neuropsychopharmacol. 2013;23(7):709–20. https://doi.org/10.1016/j.euroneuro.2012.05.012.
López-Muñoz F, Alamo C. Active metabolites as antidepressant drugs: the role of norquetiapine in the mechanism of action of quetiapine in the treatment of mood disorders. Front Psychiatry. 2013;12(4):102. https://doi.org/10.3389/fpsyt.2013.00102.
Pergola G, Selvaggi P, Trizio S, Bertolino A, Blasi G. The role of the thalamus in schizophrenia from a neuroimaging perspective. Neurosci Biobehav Rev. 2015;54:57–75. https://doi.org/10.1016/j.neubiorev.2015.01.013.
Khan MA, Al-Jahdali H. The consequences of sleep deprivation on cognitive performance. Neurosciences (Riyadh). 2023;28(2):91–9. https://doi.org/10.17712/nsj.2023.2.20220108.
Sherman SM, Guillery RW. Distinct functions for direct and transthalamic corticocortical connections. J Neurophysiol. 2011;106(3):1068–77. https://doi.org/10.1152/jn.00429.2011.
Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology. 2008;199(3):331–88. https://doi.org/10.1007/s00213-008-1072-4.
Mena A, Ruiz-Salas JC, Puentes A, Dorado I, Ruiz-Veguilla M, De la Casa LG. Reduced prepulse inhibition as a biomarker of schizophrenia. Front Behav Neurosci. 2016;18(10):202. https://doi.org/10.3389/fnbeh.2016.00202.
Carli M, Invernizzi RW. Serotoninergic and dopaminergic modulation of cortico-striatal circuit in executive and attention deficits induced by NMDA receptor hypofunction in the 5-choice serial reaction time task. Front Neural Circuits. 2014;11(8):58. https://doi.org/10.3389/fncir.2014.00058.
Liu YP, Tung CS, Chuang CH, Lo SM, Ku YC. Tail-pinch stress and REM sleep deprivation differentially affect sensorimotor gating function in modafinil-treated rats. Behav Brain Res. 2011;219:98–104. https://doi.org/10.1016/j.bbr.2010.12.012.
Frau R, Orrù M, Puligheddu M, Gessa GL, Mereu G, Marrosu F, Bortolato M. Sleep deprivation disrupts prepulse inhibition of the startle reflex: reversal by antipsychotic drugs. Int J Neuropsychopharmacol. 2008;11(7):947–55. https://doi.org/10.1017/S1461145708008900.
Machado RB, Hipólide DC, Benedito-Silva AA, Tufik S. Sleep deprivation induced by the modified multiple platform technique: quantification of sleep loss and recovery. Brain Res. 2004;1004(1–2):45–51. https://doi.org/10.1016/j.brainres.2004.01.019.
Uzbay T, Kayir H, Goktalay G, Yildirim M. Agmatine disrupts prepulse inhibition of acoustic startle reflex in rats. J Psychopharmacol. 2010;24(6):923–9. https://doi.org/10.1177/0269881109102533.
Öz P, Gökalp HK, Göver T, Uzbay T. Dose-dependent and opposite effects of orexin A on prepulse inhibition response in sleep-deprived and non-sleep-deprived rats. Behav Brain Res. 2018;2(346):73–9. https://doi.org/10.1016/j.bbr.2017.12.002.
Kaya-Yertutanol FD, Uzbay İT, Çevreli B, et al. Effect of gabapentin on sleep-deprivation-induced disruption of prepulse inhibition. Psychopharmacology. 2020;237:2993–3006. https://doi.org/10.1007/s00213-020-05587-9.
Tekin M, Kaya-Yertutanol FD, Çevreli B, Özdoğru AA, Kulaksız H, Uzbay İT. Sodium valproate improves sensorimotor gating deficit induced by sleep deprivation at low doses. Turk J Med Sci. 2021;51(3):1521–30. https://doi.org/10.3906/sag-2011-229.
Zubedat S, Freed Y, Eshed Y, Cymerblit-Sabba A, Ritter A, Nachmani M, Harush R, Aga-Mizrachi S, Avital A. Plant-derived nanoparticle treatment with cocc 30c ameliorates attention and motor abilities in sleep-deprived rats. Neuroscience. 2013;3(253):1–8. https://doi.org/10.1016/j.neuroscience.2013.08.021.
Gründer G, Heinze M, Cordes J, Mühlbauer B, Juckel G, Schulz C, Rüther E, Timm J, NeSSy Study Group. Effects of first-generation antipsychotics versus second-generation antipsychotics on quality of life in schizophrenia: a double-blind, randomised study. Lancet Psychiatry. 2016;3(8):717–29. https://doi.org/10.1016/S2215-0366(16)00085-7.
Powell SB, Young JW, Ong JC, Caron MG, Geyer MA. Atypical antipsychotics clozapine and quetiapine attenuate prepulse inhibition deficits in dopamine transporter knockout mice. Behav Pharmacol. 2008;19(5–6):562–5. https://doi.org/10.1097/FBP.0b013e32830dc110.
He J, Zu Q, Wen C, Liu Q, You P, Li X, Wang W. Quetiapine attenuates schizophrenia-like behaviors and demyelination in a MK-801-induced mouse model of schizophrenia. Front Psychiatry. 2020;19(11):843. https://doi.org/10.3389/fpsyt.2020.00843.
Chamera K, Curzytek K, Kamińska K, Trojan E, Basta-Kaim A. Quetiapine ameliorates MIA-induced impairment of sensorimotor gating: focus on neuron-microglia communication and the inflammatory response in the frontal cortex of adult offspring of Wistar rats. Cells. 2022;11(18):2788. https://doi.org/10.3390/cells11182788.
Tanibuchi Y, Fujita Y, Horio M, Iyo M, Hashimoto K. Effects of quetiapine on dizocilpine-induced prepulse inhibition deficits in mice possible role of the aα1 adrenergic receptor. Clin Psychopharmacol Neurosci. 2010;8(3):133–6.
Duncan GE, Moy SS, Lieberman JA, Koller BH. Effects of haloperidol, clozapine, and quetiapine on sensorimotor gating in a genetic model of reduced NMDA receptor function. Psychopharmacology. 2006;184(2):190–200. https://doi.org/10.1007/s00213-005-0214-1.
Li M, He E, Volf N. Time course of the attenuation effect of repeated antipsychotic treatment on prepulse inhibition disruption induced by repeated phencyclidine treatment. Pharmacol Biochem Behav. 2011;98(4):559–69. https://doi.org/10.1016/j.pbb.2011.03.007.
Shoemaker JM, Pitcher L, Noh HR, Swerdlow NR. Quetiapine produces a prolonged reversal of the sensorimotor gating-disruptive effects of basolateral amygdala lesions in rats. Behav Neurosci. 2003;117(1):136–43. https://doi.org/10.1037//0735-7044.117.1.136.
Auclair AL, Galinier A, Besnard J, Newman-Tancredi A, Depoortère R. Putative antipsychotics with pronounced agonism at serotonin 5-HT1A and partial agonist activity at dopamine D2 receptors disrupt basal PPI of the startle reflex in rats. Psychopharmacology. 2007;193(1):45–54. https://doi.org/10.1007/s00213-007-0762-7.
Molina V, López DE, Villa R, Pérez J, Martín C, Ballesteros A, Cardoso A, Sancho C. Prepulse inhibition of the startle reflex in schizophrenia remains stable with short-term quetiapine. Eur Psychiatry. 2011;26(5):271–5. https://doi.org/10.1016/j.eurpsy.2010.03.002.
Aggernaes B, Glenthoj BY, Ebdrup BH, Rasmussen H, Lublin H, Oranje B. Sensorimotor gating and habituation in antipsychotic-naive, first-episode schizophrenia patients before and after 6 months’ treatment with quetiapine. Int J Neuropsychopharmacol. 2010;13(10):1383–95. https://doi.org/10.1017/S1461145710000787.
Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology. 2001;156(2–3):117–54. https://doi.org/10.1007/s002130100811.
Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27–31. https://doi.org/10.4103/0976-0105.177703.
Christie MA, McKenna JT, Connolly NP, McCarley RW, Strecker RE. 24 hours of sleep deprivation in the rat increases sleepiness and decreases vigilance: introduction of the rat-psychomotor vigilance task. J Sleep Res. 2008;17(4):376–84. https://doi.org/10.1111/j.1365-2869.2008.00698.x.
Libourel PA, Corneyllie A, Luppi PH, Chouvet G, Gervasoni D. Unsupervised online classifier in sleep scoring for sleep deprivation studies. Sleep. 2015;38(5):815–28. https://doi.org/10.5665/sleep.4682.
Bhopal N, Khatwa U. Sleep deprivation and human development. In: Bianchi MT, editor. Sleep deprivation and disease: effects on the body, brain and behavior. New York: Springer Science Business Media; 2014. p. 91–9.
Claverie D, Becker C, Ghestem A, Coutan M, Camus F, Bernard C, Benoliel JJ, Canini F. Low β2 main peak frequency in the electroencephalogram signs vulnerability to depression. Front Neurosci. 2016;2(10):495. https://doi.org/10.3389/fnins.2016.00495.
Acknowledgements
The authors dedicated this publication to the 100th anniversary of the Republic of Türkiye. As scientists raised by Türkiye, we are proud of to be citizen of this country.
Funding
Funded by Üsküdar University Scientific Research Projects Unit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Ethical approval
All experiments were conducted according to the ethical guidelines in the Guidelines for the Care and Use of Laboratory Animals adopted by the US National Institutes of Health and published in 1996 and OECD guidelines no. 423. All animal experiments were performed with prior permission from the Animal Research Ethics Committee of Üsküdar University, İstanbul, Turkey. This study was approved by the Local Ethic Committee of Uskudar University on February 20.01.2023 the decision number of Ü.Ü-HADYEK 2023–03.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Özcan, Ö.Ö., Çevreli, B., Temizyürek, A. et al. Quetiapine improves sensorimotor gating deficit in a sleep deprivation-induced rat model. Sleep Biol. Rhythms 22, 269–278 (2024). https://doi.org/10.1007/s41105-023-00504-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41105-023-00504-x