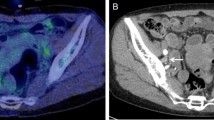
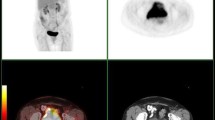
Abstract
Objectives
To evaluate the diagnostic performance of contrast-enhanced F18 FDG PET-CT with conventional modality (contrast-enhanced computed tomography) in restaging of cervical carcinoma.
Study Design
A retrospective analysis of histopathological proven patients of carcinoma cervix (n = 386; age range 32–82 years; mean age 51.4 years) between April 2017 and July 2022 was done. All the patients were treated either in combination or alone with surgery, radiotherapy or chemotherapy. The patients were referred for F18 FDG PET-CT on the basis of clinical and imaging suspicion of residual or recurrent disease and for post-therapy monitoring. Histopathological examination and clinical or imaging follow-up were taken as gold standard.
Results
Of 386 patients, underwent PET-CT examination for post-therapy monitoring. F18 FDG PET-CT detected abnormal FDG uptake in 258 patients. Uptake at the primary site was detected in 112/258 (43.41%) patients, and only regional pelvic lymph nodes and distant metastatic lesions were in 18/258 (~ 6.96%) and 128/258 (49.4%) patients, respectively. On lesion-based analyses, distant metastases, lymph nodes, lung, liver and skeleton were noted in 128, 50, 18 and 30 patients respectively, either isolated or combined. Fourteen patients had distant metastases at rare sites including kidney, muscle, ascites, pleural effusion and gut (4, 4, 2, 2, 2). Additionally, metachronous primaries were also detected in 18 patients and confirmed on histopathology (lung-6, pharynx-4, breast-4, lymphoma-2 and rectum-2). After a median follow-up period of 30 months (± SD 20.8), 226 patients having residual or recurrence disease and FDG PET-CT had the sensitivity, specificity, PPV, NPV and accuracy as 89.38, 65, 78.29, 81.25 and 80%, respectively, while conventional imaging (CI) had the sensitivity, specificity, PPV, NPV and accuracy of 66.42, 40.68, 71.77, 34.78 and 70%, respectively. On ROC curve analysis, SUVmax > 4.4 had sensitivity and specificity 78.8 and 83.7%, respectively, for lesion detection on F18 FDG PET-CT. There was a significant correlation between SUVmax and patient outcome (r = ~ 0.0585).
Conclusions
This study concluded that F18 FDG PET-CT has high diagnostic accuracy as compared to conventional imaging for detection of suspected residual/recurrent disease in cervical cancer patients and also useful in detection of additional metachronous second primary.






Similar content being viewed by others
Data availability
Contact the corresponding author for data requests.
References
Bray F, Ferlay J, Soerjomataram I, Siegel R, Torre LA, Jemal A. Global incidence statistics 2018: GLOBOCAN: estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2018;68:394–424.
Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, et al. Human papilloma-virus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–9. https://doi.org/10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431%3e3.0.CO;2-F.
Bhatla N, Berek JS, Cuello Fredes M, et al. Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynaecol Obstet. 2019;145(1):129–35. https://doi.org/10.1002/ijgo.12749.
Liu FY, Lai CH, Yang LY, Wang CC, Lin G, et al. Utility of (18)F-FDG PET/CT in patients with advanced squamous cell carcinoma of the uterine cervix receiving concurrent chemoradiotherapy: a parallel study of a prospective randomized trial. Eur J Nucl Med Mol Imaging. 2016;43:1812–23. https://doi.org/10.1007/s00259-016-3384-7.
Gadducci A, Teti G, Barsotti C, et al. Clinicopathological variables predictive of clinical outcome in patients with FIGO stage Ib2-IIb cervical cancer treated with cisplatin-based neoadjuvant chemotherapy followed by radical hysterectomy. Anticancer Res. 2010;30(1):201–8.
Hong JH, Tsai CS, Lai CH, et al. Recurrent squamous cell carcinoma of cervix after definitive radiotherapy. Int J Radiat Oncol Biol Phys. 2004;60(1):249–57. https://doi.org/10.1016/j.ijrobp.2004.02.044.
Eifel PJ, Winter K, Morris M, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90–01. J Clin Oncol. 2004;22(5):872–80. https://doi.org/10.1200/JCO.2004.07.197.
Wang H, Zhu L, Lu W, Xu H, Yu Y, Yang Y. Clinicopathological risk factors for recurrence after neoadjuvant chemotherapy and radical hysterectomy in cervical cancer. World J Surg Oncol. 2013;11:301. https://doi.org/10.1186/1477-7819-11-301.
Kubota H, Tsujino K, Sulaiman NS, et al. Comparison of salvage therapies for isolated para-aortic lymph node recurrence in patients with uterine cervical cancer after definitive treatment. Radiat Oncol. 2019;14(1):236. https://doi.org/10.1186/s13014-019-1442-6.
Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the cervix uteri. Int J Gynaecol Obstet. 2018;143(Suppl 2):22–36. https://doi.org/10.1002/ijgo.12611.
Gadducci A, Tana R, Cosio S, Genazzani AR. The serum assay of tumour markers in the prognostic evaluation, treatment monitoring and follow-up of patients with cervical cancer: a review of the literature. Crit Rev Oncol Hematol. 2008;66(1):10–20. https://doi.org/10.1016/j.critrevonc.2007.09.002.
Torizuka T, Nobezawa S, Kanno T, et al. Ovarian cancer recurrence: role of whole-body positron emission tomography using 2-[fluorine-18]-fluoro-2-deoxy- D-glucose. Eur J Nucl Med Mol Imaging. 2002;29(6):797–803. https://doi.org/10.1007/s00259-001-0750-9.
Yildirim Y, Sehirali S, Avci ME, et al. Integrated PET/CT for the evaluation of para-aortic nodal metastasis in locally advanced cervical cancer patients with negative conventional CT findings. Gynecol Oncol. 2008;108(1):154–9. https://doi.org/10.1016/j.ygyno.2007.09.011.
Lin G, Lai CH, Yen TC. Emerging molecular imaging techniques in gynecologic oncology. PET Clin. 2018;13(2):289–99. https://doi.org/10.1016/j.cpet.2017.11.011.
Kidd EA, Siegel BA, Dehdashti F, Grigsby PW. The standardized uptake value for F-18 fluorodeoxyglucose is a sensitive predictive biomarker for cervical cancer treatment response and survival. Cancer. 2007;110(8):1738–44. https://doi.org/10.1002/cncr.22974.
Gold MA. PET in cervical cancer–implications for “staging”, treatment planning, assessment of prognosis, and prediction of response. J Natl Compr Canc Netw. 2008;6(1):37–45. https://doi.org/10.6004/jnccn.2008.0004.
Scarsbrook A, Vaidyanathan S, Chowdhury F, Swift S, Cooper R, Patel C. Efficacy of qualitative response assessment interpretation criteria at 18F-FDG PET-CT for predicting outcome in locally advanced cervical carcinoma treated with chemoradiotherapy. Eur J Nucl Med Mol Imaging. 2017;44(4):581–8. https://doi.org/10.1007/s00259-016-3537-8.
Chong A, Ha JM, Jeong SY, Song HC, Min JJ, Bom HS, Choi HS. Clinical usefulness of (18)F-FDG PET/CT in the detection of early recurrence in treated cervical cancer patients with unexplained elevation of serum tumor markers. Chonnam Med J. 2013;49(1):20–6. https://doi.org/10.4068/cmj.2013.49.1.20.
Ryu SY, Kim MH, Choi SC, Choi CW, Lee KH. Detection of early recurrence with 18F-FDG PET in patients with cervical cancer. J Nucl Med. 2003;44(3):347–52.
Yen TC, See LC, Chang TC, et al. Defining the priority of using 18F-FDG PET for recurrent cervical cancer. J Nucl Med. 2004;45(10):1632–9.
Magné N, Chargari C, Vicenzi L, et al. New trends in the evaluation and treatment of cervix cancer: the role of FDG-PET. Cancer Treat Rev. 2008;34(8):671–81. https://doi.org/10.1016/j.ctrv.2008.08.003.
Sun SS, Chen TC, Yen RF, Shen YY, Changlai SP, Kao A. Value of whole body 18F-fluoro-2-deoxyglucose positron emission tomography in the evaluation of recurrent cervical cancer. Anticancer Res. 2001;21(4B):2957–61.
Lai CH, Huang KG, See LC, et al. Restaging of recurrent cervical carcinoma with dual-phase [18F]fluoro-2-deoxy-D-glucose positron emission tomography. Cancer. 2004;100(3):544–52. https://doi.org/10.1002/cncr.11928.
Havrilesky LJ, Wong TZ, Secord AA, Berchuck A, Clarke-Pearson DL, Jones EL. The role of PET scanning in the detection of recurrent cervical cancer. Gynecol Oncol. 2003;90(1):186–90. https://doi.org/10.1016/s0090-8258(03)00256-7.
Chang TC, Law KS, Hong JH, et al. Positron emission tomography for unexplained elevation of serum squamous cell carcinoma antigen levels during follow-up for patients with cervical malignancies: a phase II study. Cancer. 2004;101(1):164–71. https://doi.org/10.1002/cncr.20349.
Unger JB, Ivy JJ, Connor P, Charrier A, Ramaswamy MR, Ampil FL, Monsour RP. Detection of recurrent cervical cancer by whole-body FDG PET scan in asymptomatic and symptomatic women. Gynecol Oncol. 2004;94(1):212–6. https://doi.org/10.1016/j.ygyno.2004.04.021.
Chung HH, Jo H, Kang WJ, et al. Clinical impact of integrated PET/CT on the management of suspected cervical cancer recurrence. Gynecol Oncol. 2007;104(3):529–34. https://doi.org/10.1016/j.ygyno.2006.09.009.
Kitajima K, Murakami K, Yamasaki E, Domeki Y, Kaji Y, Sugimura K. Performance of FDG-PET/CT for diagnosis of recurrent uterine cervical cancer. Eur Radiol. 2008;18(10):2040–7. https://doi.org/10.1007/s00330-008-0979-9.
Mittra E, El-Maghraby T, Rodriguez CA, et al. Efficacy of 18F-FDG PET/CT in the evaluation of patients with recurrent cervical carcinoma. Eur J Nucl Med Mol Imaging. 2009;36(12):1952–9. https://doi.org/10.1007/s00259-009-1206-x.
Hirakawa M, Nagai Y, Toita T, et al. High-risk group for locoregional recurrence in patients with stage IB-IIB squamous cell carcinoma of the cervix treated with concurrent chemoradiotherapy. Anticancer Res. 2011;31(4):1437–41.
Bhandari V, Kausar M, Naik A, Batra M. Unusual metastasis from carcinoma cervix. J Obstet Gynaecol India. 2016;66(5):358–62. https://doi.org/10.1007/s13224-015-0692-y.
Arnold M, Liu L, Kenter GG, Creutzberg CL, Coebergh JW, Soerjomataram I. Second primary cancers in survivors of cervical cancer in The Netherlands: implications for prevention and surveillance. Radiother Oncol. 2014;111(3):374–81. https://doi.org/10.1016/j.radonc.2014.04.011.
Chaturvedi AK, Engels EA, Gilbert ES, et al. Second cancers among 104,760 survivors of cervical cancer: evaluation of long-term risk. J Natl Cancer Inst. 2007;99(21):1634–43. https://doi.org/10.1093/jnci/djm201.
Chen CY, Lai CH, Lee KD, Huang SH, Dai YM, Chen MC. Risk of second primary malignancies in women with cervical cancer: a population-based study in Taiwan over a 30-year period. Gynecol Oncol. 2012;127(3):625–30. https://doi.org/10.1016/j.ygyno.2012.09.004.
Choi J, Kim HJ, Jeong YH, et al. The role of (18) F-FDG PET/CT in assessing therapy response in cervix cancer after concurrent chemoradiation therapy. Nucl Med Mol Imaging. 2014;48(2):130–6. https://doi.org/10.1007/s13139-013-0248-y.
Chung HH, Kim JW, Kang KW, et al. Predictive role of post-treatment [18F]FDG PET/CT in patients with uterine cervical cancer. Eur J Radiol. 2012;81(8):e817–22. https://doi.org/10.1016/j.ejrad.2012.02.015.
Acknowledgements
I would like to thank our supporting staff (Technician and nursing staff) members and supporting doctors who help me a lot during data acquisition, patient follow-up and image interpretation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
This study is approved by institutional ethical committee. All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
This retrospective study was approved by the institutional ethics committee. In view of the retrospective study design, written informed consent was waived.
Informed consent
Informed consent was obtained from individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jain, T.K., Agrawal, N., Malhotra, H. et al. Is F18 FDG PET-CT an Authoritative Investigation as Compared to Other Conventional Imaging (CECT) Modality in Treated Cervical Cancer Patients?. Indian J Gynecol Oncolog 21, 51 (2023). https://doi.org/10.1007/s40944-023-00731-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40944-023-00731-7