Abstract
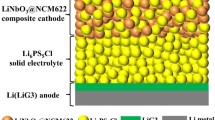
Sulfide solid electrolyte is a promising candidate for the development of high-energy lithium-sulfur (Li-S) batteries. However, the concurrent improvement of ionic conductivity, bulk air stability, and compatibility of the electrolyte/electrode interface of sulfide solid electrolyte remains a huge challenge. Herein, we propose a dual-source doping (Sb2O3 and LiI) strategy to prepare a multifunctional sulfide solid electrolyte. Sb2O3 can broaden the transmission path of lithium ions and improve the bulk stability, and LiI can inhibit the generation of lithium dendrites and reduce the electrolyte/electrode resistance. Therefore, the sulfide solid electrolyte can be strengthened in terms of its bulk and interface, thus exhibiting a high ionic conductivity of 1.69 × 10−3 S cm−1 at 30°C, high air stability, and high electrochemical stability with lithium metal. On this basis, the as-prepared all-solid-state Li-S batteries (ASSLSBs) can exhibit a high specific discharge capacity after being cycled at 0.05 C for 100 cycles at room temperature (833 mA h g−1) or 60°C (949 mA h g−1). This work provides a rational scheme for the preparation of practical sulfide solid electrolytes and high-performance ASSLSBs.

摘要
硫化物固体电解质是发展高容量锂硫电池的理想候选者. 然而, 同时提高硫化物固体电解质的离子导电性、空气稳定性和电解质/电 极界面的相容性仍然是一个巨大的挑战. 因此, 我们提出了一种双掺杂 (Sb2O3和LiI)策略来制备多功能硫化物固体电解质. Sb2O3可以拓宽锂离 子的传输路径和提高空气稳定性, 而LiI可以抑制锂枝晶的生成和降低 电解质/电极之间的电阻. 因此, 硫化物固体电解质在空气中和界面上 的性能得到了增强, 在30°C下的离子电导率为1.69 × 10−3 S cm−1, 且具 有很好的空气稳定性, 对金属锂也很稳定. 在此基础上, 组装的全固态 锂硫电池以0.05 C循环100圈后, 表现出较高的放电比容量(室温, 833 mA h g−1; 60°C: 949 mA h g−1). 本文为制备实用的硫化物固体电解 质和高性能全固态锂硫电池提供了合理的方案.
Similar content being viewed by others
References
Ji X, Lee KT, Nazar LF. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nat Mater, 2009, 8: 500–506
Yin YX, Xin S, Guo YG, et al. Lithium-sulfur batteries: Electrochemistry, materials, and prospects. Angew Chem Int Ed, 2013, 52: 13186–13200
Zhou W, Guo B, Gao H, et al. Low-cost higher loading of a sulfur cathode. Adv Energy Mater, 2016, 6: 1502059
Fan L, Chen S, Ma R, et al. Ultrastable potassium storage performance realized by highly effective solid electrolyte interphase layer. Small, 2018, 14: 1801806
Park SW, Oh G, Park JW, et al. Graphitic hollow nanocarbon as a promising conducting agent for solid-state lithium batteries. Small, 2019, 15: 1900235
Fitzhugh W, Wu F, Ye L, et al. Strain-stabilized ceramic-sulfide electrolytes. Small, 2019, 15: 1901470
Shen Z, Zhang W, Zhu G, et al. Design principles of the anode-electrolyte interface for all solid-state lithium metal batteries. Small Methods, 2019, 4: 1900592
Han F, Yue J, Fan X, et al. High-performance all-solid-state lithium-sulfur battery enabled by a mixed-conductive Li2S nanocomposite. Nano Lett, 2016, 16: 4521–4527
Yan H, Wang H, Wang D, et al. In situ generated Li2S-C nano-composite for high-capacity and long-life all-solid-state lithium sulfur batteries with ultrahigh areal mass loading. Nano Lett, 2019, 19: 3280–3287
Xu R, Yue J, Liu S, et al. Cathode-supported all-solid-state lithium-sulfur batteries with high cell-level energy density. ACS Energy Lett, 2019, 4: 1073–1079
Albertus P, Babinec S, Litzelman S, et al. Status and challenges in enabling the lithium metal electrode for high-energy and low-cost rechargeable batteries. Nat Energy, 2018, 3: 16–21
Zhang K, Lee GH, Park M, et al. Recent developments of the lithium metal anode for rechargeable non-aqueous batteries. Adv Energy Mater, 2016, 6: 1600811
Li S, Zhang W, Wu Q, et al. Synergistic dual-additive electrolyte enables practical lithium-metal batteries. Angew Chem Int Ed, 2020, 59: 14935–14941
Zhao Q, Liu X, Stalin S, et al. Solid-state polymer electrolytes with inbuilt fast interfacial transport for secondary lithium batteries. Nat Energy, 2019, 4: 365–373
Wang S, Bai Q, Nolan AM, et al. Lithium chlorides and bromides as promising solid-state chemistries for fast ion conductors with good electrochemical stability. Angew Chem Int Ed, 2019, 58: 8039–8043
Zhang Q, Cao D, Ma Y, et al. Sulfide-based solid-state electrolytes: Synthesis, stability, and potential for all-solid-state batteries. Adv Mater, 2019, 31: 1901131
Wu J, Liu S, Han F, et al. Lithium/sulfide all-solid-state batteries using sulfide electrolytes. Adv Mater, 2021, 33: 2000751
Wu J, Shen L, Zhang Z, et al. All-solid-state lithium batteries with sulfide electrolytes and oxide cathodes. Electrochem Energ Rev, 2021, 4: 101–135
Liu G, Shi J, Zhu M, et al. Ultra-thin free-standing sulfide solid electrolyte film for cell-level high energy density all-solid-state lithium batteries. Energy Storage Mater, 2021, 38: 249–254
Zhang Z, Wu L, Zhou D, et al. Flexible sulfide electrolyte thin membrane with ultrahigh ionic conductivity for all-solid-state lithium batteries. Nano Lett, 2021, 21: 5233–5239
Mizuno F, Hayashi A, Tadanaga K, et al. New, highly ion-conductive crystals precipitated from Li2S-P2S5 glasses. Adv Mater, 2005, 17: 918–921
Zhang Z, Chen S, Yang J, et al. Interface re-engineering of Li10GeP2S12 electrolyte and lithium anode for all-solid-state lithium batteries with ultralong cycle life. ACS Appl Mater Interfaces, 2018, 10: 2556–2565
Liu G, Xie D, Wang X, et al. High air-stability and superior lithium ion conduction of Li3+3xP1−xZnxS4−xOx by aliovalent substitution of ZnO for all-solid-state lithium batteries. Energy Storage Mater, 2019, 17: 266–274
Liu G, Weng W, Zhang Z, et al. Densified Li6PS5Cl nanorods with high ionic conductivity and improved critical current density for all-solidstate lithium batteries. Nano Lett, 2020, 20: 6660–6665
Chen S, Xie D, Liu G, et al. Sulfide solid electrolytes for all-solid-state lithium batteries: Structure, conductivity, stability and application. Energy Storage Mater, 2018, 14: 58–74
Seino Y, Ota T, Takada K, et al. A sulphide lithium super ion conductor is superior to liquid ion conductors for use in rechargeable batteries. Energy Environ Sci, 2014, 7: 627–631
Su D, Zhou D, Wang C, et al. Toward high performance lithium-sulfur batteries based on Li2S cathodes and beyond: Status, challenges, and perspectives. Adv Funct Mater, 2018, 28: 1800154
Zhang Z, Zhang L, Yan X, et al. All-in-one improvement toward Li6PS5Br-based solid electrolytes triggered by compositional tune. J Power Sources, 2019, 410–411: 162–170
Zhao BS, Wang L, Chen P, et al. Congener substitution reinforced Li7P2.9Sb0.1S10.75O0.25 glass-ceramic electrolytes for all-solid-state lithium-sulfur batteries. ACS Appl Mater Interfaces, 2021, 13: 34477–34485
Garcia-Mendez R, Mizuno F, Zhang R, et al. Effect of processing conditions of 75Li2S-25P2S5 solid electrolyte on its DC electrochemical behavior Electrochim Acta, 2017, 237: 144–151
Han F, Zhu Y, He X, et al. Electrochemical stability of Li10GeP2S12 and Li7La3Zr2O12 solid electrolytes. Adv Energy Mater, 2016, 6: 1501590
Ohtomo T, Hayashi A, Tatsumisago M, et al. Characteristics of the Li2O-Li2S-P2S5 glasses synthesized by the two-step mechanical milling. J Non-Crystalline Solids, 2013, 364: 57–61
Li X, Liang J, Chen N, et al. Water-mediated synthesis of a superionic halide solid electrolyte. Angew Chem Int Ed, 2019, 58: 16427–16432
Chen T, Zhang L, Zhang Z, et al. Argyrodite solid electrolyte with a stable interface and superior dendrite suppression capability realized by ZnO co-doping. ACS Appl Mater Interfaces, 2019, 11: 40808–40816
Lu Y, Tu Z, Archer LA. Stable lithium electrodeposition in liquid and nanoporous solid electrolytes. Nat Mater, 2014, 13: 961–969
Ma L, Kim MS, Archer LA. Stable artificial solid electrolyte interphases for lithium batteries. Chem Mater, 2017, 29: 4181–4189
Choi SJ, Choi SH, Bui AD, et al. LiI-doped sulfide solid electrolyte: Enabling a high-capacity slurry-cast electrode by low-temperature post-sintering for practical all-solid-state lithium batteries. ACS Appl Mater Interfaces, 2018, 10: 31404–31412
Xu R, Xia X, Li S, et al. All-solid-state lithium-sulfur batteries based on a newly designed Li7P2.9Mn0.1S10.7I0.3 superionic conductor. J Mater Chem A, 2017, 5: 6310–6317
Dietrich C, Sadowski M, Sicolo S, et al. Local structural investigations, defect formation, and ionic conductivity of the lithium ionic conductor Li4P2S6. Chem Mater, 2016, 28: 8764–8773
Huang B, Yao X, Huang Z, et al. Li3PO4-doped Li7P3S11 glass-ceramic electrolytes with enhanced lithium ion conductivities and application in all-solid-state batteries J Power Sources, 2015, 284: 206–211
Xu R, Xia X, Wang X, et al. Tailored Li2S-P2S5 glass-ceramic electrolyte by MoS2 doping, possessing high ionic conductivity for all-solid-state lithium-sulfur batteries J Mater Chem A, 2017, 5: 2829–2834
Dietrich C, Weber DA, Sedlmaier SJ, et al. Lithium ion conductivity in Li2S-P2S5 glasses-building units and local structure evolution during the crystallization of superionic conductors Li3PS4, Li7P3S11 and Li4P2S7. J Mater Chem A, 2017, 5: 18111–18119
Zhou L, Tufail MK, Ahmad N, et al. Strong interfacial adhesion between the Li2S cathode and a functional Li7P2.9Ce0.2S10.9Cl0.3 solid-state electrolyte endowed long-term cycle stability to all-solid-state lithium-sulfur batteries. ACS Appl Mater Interfaces, 2021, 13: 28270–28280
Seino Y, Nakagawa M, Senga M, et al. Analysis of the structure and degree of crystallisation of 70Li2S-30P2S5 glass ceramic. J Mater Chem A, 2015, 3: 2756–2761
Wang Y, Lu D, Bowden M, et al. Mechanism of formation of Li7P3S11 solid electrolytes through liquid phase synthesis. Chem Mater, 2018, 30: 990–997
Liang J, Chen N, Li X, et al. Li10Ge(P1−xSbx)2S12 lithium-ion conductors with enhanced atmospheric stability. Chem Mater, 2020, 32: 2664–2672
Kaup K, Lalère F, Huq A, et al. Correlation of structure and fast ion conductivity in the solid solution series Li1+2xZn1−xPS4. Chem Mater, 2018, 30: 592–596
Yi J, Chen L, Liu Y, et al. High capacity and superior cyclic performances of all-solid-state lithium-sulfur batteries enabled by a high-conductivity Li10SnP2S12 solid electrolyte. ACS Appl Mater Interfaces, 2019, 11: 36774–36781
Zhang Z, Zhang J, Sun Y, et al. Li4−xSbxSn1−xS4 solid solutions for air-stable solid electrolytes. J Energy Chem, 2020, 41: 171–176
Muramatsu H, Hayashi A, Ohtomo T, et al. Structural change of Li2S-P2S5 sulfide solid electrolytes in the atmosphere. Solid State Ion, 2011, 182: 116–119
Ohtomo T, Hayashi A, Tatsumisago M, et al. Suppression of H2S gas generation from the 75Li2S-25P2S5 glass electrolyte by additives. J Mater Sci, 2013, 48: 4137–4142
Sahu G, Rangasamy E, Li J, et al. A high-conduction Ge substituted Li3AsS4 solid electrolyte with exceptional low activation energy. J Mater Chem A, 2014, 2: 10396–10403
Ahmad N, Zhou L, Faheem M, et al. Enhanced air stability and high Li-ion conductivity of Li6.988P2.994Nb0.2S10.934O0.6 glass-ceramic electrolyte for all-solid-state lithium-sulfur batteries. ACS Appl Mater Interfaces, 2020, 12: 21548–21558
Hayashi A, Muramatsu H, Ohtomo T, et al. Improvement of chemical stability of Li3PS4 glass electrolytes by adding MxOy (M = Fe, Zn, and Bi) nanoparticles. J Mater Chem A, 2013, 1: 6320–6326
Park M, Jung HG, Jung WD, et al. Chemically evolved composite lithium-ion conductors with lithium thiophosphates and nickel sulfides. ACS Energy Lett, 2017, 2: 1740–1745
Zhang Y, Chen R, Liu T, et al. High capacity, superior cyclic performances in all-solid-state lithium-ion batteries based on 78Li2S-22P2S5 glass-ceramic electrolytes prepared via simple heat treatment. ACS Appl Mater Interfaces, 2017, 9: 28542–28548
Zhou L, Tufail MK, Yang L, et al. Cathode-doped sulfide electrolyte strategy for boosting all-solid-state lithium batteries. Chem Eng J, 2020, 391: 123529
Liu H, Cheng XB, Huang JQ, et al. Controlling dendrite growth in solid-state electrolytes. ACS Energy Lett, 2020, 5: 833–843
Nagao M, Imade Y, Narisawa H, et al. All-solid-state Li-sulfur batteries with mesoporous electrode and thio-LISICON solid electrolyte. J Power Sources, 2013, 222: 237–242
Ma Q, Qi X, Tong B, et al. Novel Li[(CF3SO2)(n-C4F9SO2)N]-based polymer electrolytes for solid-state lithium batteries with superior electrochemical performance. ACS Appl Mater Interfaces, 2016, 8: 29705–29712
Kim KJ, Balaish M, Wadaguchi M, et al. Solid-state Li-metal batteries: Challenges and horizons of oxide and sulfide solid electrolytes and their interfaces. Adv Energy Mater, 2021, 11: 2002689
Lau J, DeBlock RH, Butts DM, et al. Sulfide solid electrolytes for lithium battery applications. Adv Energy Mater, 2018, 8: 1800933
Xu R, Zhang S, Wang X, et al. Recent developments of all-solid-state lithium secondary batteries with sulfide inorganic electrolytes. Chem Eur J, 2018, 24: 6007–6018
Acknowledgements
This work was supported by the Science and Technology Support Plan of Tianjin (19YFZCGX00220), the National Natural Science Foundation of China (21935006), and the Fundamental Research Funds for the Central Universities, Nankai University (63211043).
Author information
Authors and Affiliations
Contributions
Zhao BS, Chen P, and Gao XP conceived the idea. Zhao BS carried out the preparation and electrochemical tests of the devices. Zhao BS, Chen P, and Gao XP co-wrote the paper. All the authors contributed to the general discussion.
Corresponding authors
Additional information
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary information
Supporting data are available in the online version of the paper.
Bo-Sheng Zhao is currently a PhD candidate at the School of Materials Science and Engineering, Nankai University. His general research interests are in the areas of modifying sulfide solid electrolytes and preparing high-performance all-solid-state lithium-sulfur batteries.
Peng Chen is a lecturer at the School of Materials Science and Engineering, Nankai University. He received his BS degree in 2015 from the College of Chemistry and PhD degree in 2020 from the School of Materials Science and Engineering, Nankai University. His general research interests are in the area of novel energy conversion and storage systems, including solar rechargeable batteries, new types of solar cells, and all-solid-state batteries.
Xue-Ping Gao is a professor at the Institute of New Energy Material Chemistry, Nankai University. He received his doctorate degree from the Department of Chemistry at Nankai University in 1995. He worked as a visiting research fellow at Kogakuin University in Japan from 1997 to 1999. Currently, his main research focuses on energy storage materials for power sources, including lithium-ion, lithium-sulfur, and solar rechargeable batteries.
Supplementary Information
40843_2022_2182_MOESM1_ESM.pdf
Bulk and interface-strengthened Li7P2.9Sb0.1S10.65O0.15I0.2 electrolyte via dual-source doping for all-solid-state lithium-sulfur batteries
Rights and permissions
About this article
Cite this article
Zhao, BS., Chen, P. & Gao, XP. Bulk and interface-strengthened Li7P2.9Sb0.1S10.65O0.15I0.2 electrolyte via dual-source doping for all-solid-state lithium-sulfur batteries. Sci. China Mater. 66, 513–521 (2023). https://doi.org/10.1007/s40843-022-2182-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-022-2182-0