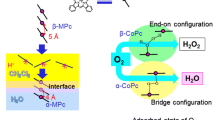
Abstract
The demand for new catalysts for renewable energy production has become crucial. As an alternative to metal catalysts for electrocatalysis to produce energy sources, metal-phthalocyanine (MPc) electrocatalysts have shown potential. Their physicochemical and electrochemical properties depend on the chemical structure of the MPc and the central metal atom. Recent reviews of MPcs focused on their electrochemical performance in specific catalytic reactions, such as oxygen reduction reaction and CO2 reduction reaction. However, understanding the structure of MPcs in depth is important, since their electrochemical catalytic activity is affected by structural modifications of MPcs. Therefore, this minireview focuses on how the molecular structure of MPcs affects electrochemical catalysis.

Similar content being viewed by others
References
Nørskov JK, Bligaard T, Logadottir A, et al. Trends in the exchange current for hydrogen evolution. J Electrochem Soc, 2005, 152: J23
Greeley J, Stephens IEL, Bondarenko AS, et al. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat Chem, 2009, 1: 552–556
Shao M, Chang Q, Dodelet JP, et al. Recent advances in electrocatalysts for oxygen reduction reaction. Chem Rev, 2016, 116: 3594–3657
Lee Y, Suntivich J, May KJ, et al. Synthesis and activities of rutile IrO2 and RuO2 nanoparticles for oxygen evolution in acid and alkaline solutions. J Phys Chem Lett, 2012, 3: 399–404
McCrory CCL, Jung S, Peters JC, et al. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J Am Chem Soc, 2013, 135: 16977–16987
Loiudice A, Lobaccaro P, Kamali EA, et al. Tailoring copper nanocrystals towards C2 products in electrochemical CO2 reduction. Angew Chem Int Ed, 2016, 55: 5789–5792
Goyal A, Marcandalli G, Mints VA, et al. Competition between CO2 reduction and hydrogen evolution on a gold electrode under well-defined mass transport conditions. J Am Chem Soc, 2020, 142: 4154–4161
Jasinski R. A new fuel cell cathode catalyst. Nature, 1964, 201: 1212–1213
Zagal J, Páez M, Tanaka AA, et al. Electrocatalytic activity of metal phthalocyanines for oxygen reduction. J Electroanal Chem, 1992, 339: 13–30
Jiang J. Functional Phthalocyanine Molecular Materials. Berlin: Springer, 2010. 135
Yang S, Yu Y, Gao X, et al. Recent advances in electrocatalysis with phthalocyanines. Chem Soc Rev, 2021, 50: 12985–13011
Mack J, Stillman MJ. Assignment of the optical spectra of metal phthalocyanines through spectral band deconvolution analysis and ZINDO calculations. Coord Chem Rev, 2001, 219–221: 993–1032
Lin L, Li H, Yan C, et al. Synergistic catalysis over iron-nitrogen sites anchored with cobalt phthalocyanine for efficient CO2 electroreduction. Adv Mater, 2019, 31: 1903470
Yilmaz I, Arslan S, Guney S, et al. Synthesis, electro-spectroelectrochemical characterization and electrocatalytic behavior towards dioxygen reduction of a new water-soluble cobalt phthalocyanine containing naphthoxy-4-sulfonic acid sodium salt. Electrochim Acta, 2007, 52: 6611–6621
Choi J, Wagner P, Gambhir S, et al. Steric modification of a cobalt phthalocyanine/graphene catalyst to give enhanced and stable electrochemical CO2 reduction to CO. ACS Energy Lett, 2019, 4: 666–672
Zhang W, Meeus EJ, Wang L, et al. Boosting electrochemical oxygen reduction performance of iron phthalocyanine through axial coordination sphere interaction. ChemSusChem, 2022, 15: 202102379
Li Z, Zhuang Z, Lv F, et al. The marriage of the FeN4 moiety and MXene boosts oxygen reduction catalysis: Fe 3d electron delocalization matters. Adv Mater, 2018, 30: 1803220
Feng S, Luo N, Tang A, et al. Phthalocyanine and metal phthalocyanines adsorbed on graphene: A density functional study. J Phys Chem C, 2019, 123: 16614–16620
Wang X, Wang B, Zhong J, et al. Iron polyphthalocyanine sheathed multiwalled carbon nanotubes: A high-performance electrocatalyst for oxygen reduction reaction. Nano Res, 2016, 9: 1497–1506
Zhang H, Zhang S, Wang Y, et al. Boosting the performance of ironphthalocyanine as cathode electrocatalyst for alkaline polymer fuel cells through edge-closed conjugation. ACS Appl Mater Interfaces, 2018, 10: 28664–28671
Nguyen D, Kang G, Chiang N, et al. Probing molecular-scale catalytic interactions between oxygen and cobalt phthalocyanine using tip-enhanced raman spectroscopy. J Am Chem Soc, 2018, 140: 5948–5954
Ince M, Yum JH, Kim Y, et al. Molecular engineering of phthalocyanine sensitizers for dye-sensitized solar cells. J Phys Chem C, 2014, 118: 17166–17170
Mori S, Nagata M, Nakahata Y, et al. Enhancement of incident photon-to-current conversion efficiency for phthalocyanine-sensitized solar cells by 3D molecular structuralization. J Am Chem Soc, 2010, 132: 4054–4055
Ma DD, Han SG, Cao C, et al. Remarkable electrocatalytic CO2 reduction with ultrahigh CO/H2 ratio over single-molecularly immobilized pyrrolidinonyl nickel phthalocyanine. Appl Catal B-Environ, 2020, 264: 118530
Li W, Yu A, Higgins DC, et al. Biologically inspired highly durable iron phthalocyanine catalysts for oxygen reduction reaction in polymer electrolyte membrane fuel cells. J Am Chem Soc, 2010, 132: 17056–17058
Li R, Zhang X, Zhu P, et al. Electron-donating or -withdrawing nature of substituents revealed by the electrochemistry of metal-free phthalocyanines. Inorg Chem, 2006, 45: 2327–2334
Boutin E, Merakeb L, Ma B, et al. Molecular catalysis of CO2 reduction: Recent advances and perspectives in electrochemical and light-driven processes with selected Fe, Ni and Co aza macrocyclic and polypyridine complexes. Chem Soc Rev, 2020, 49: 5772–5809
Cardenasjiron G. Reactivity of electrodes modified with substituted metallophthalocyanines. Correlations with redox potentials, Hammett parameters and donor-acceptor intermolecular hardness. Electrochim Acta, 2001, 46: 3227–3235
Claußen JA, Ochoa G, Páez M, et al. Volcano correlations for the reactivity of surface-confined cobalt N4-macrocyclics for the electrocatalytic oxidation of 2-mercaptoacetate. J Solid State Electrochem, 2008, 12: 473–481
Chen K, Cao M, Lin Y, et al. Ligand engineering in nickel phthalocyanine to boost the electrocatalytic reduction of CO2. Adv Funct Mater, 2022, 32: 2111322
Yang Y, Li J, Zhang C, et al. Theoretical insights into nitrogen-doped graphene-supported Fe, Co, and Ni as single-atom catalysts for CO2 reduction reaction. J Phys Chem C, 2022, 126: 4338–4346
Wu Y, Jiang Z, Lu X, et al. Domino electroreduction of CO2 to methanol on a molecular catalyst. Nature, 2019, 575: 639–642
Morlanés N, Takanabe K, Rodionov V. Simultaneous reduction of CO2 and splitting of H2O by a single immobilized cobalt phthalocyanine electrocatalyst. ACS Catal, 2016, 6: 3092–3095
Zhang X, Wu Z, Zhang X, et al. Highly selective and active CO2 reduction electrocatalysts based on cobalt phthalocyanine/carbon nanotube hybrid structures. Nat Commun, 2017, 8: 14675
Wang M, Torbensen K, Salvatore D, et al. CO2 electrochemical catalytic reduction with a highly active cobalt phthalocyanine. Nat Commun, 2019, 10: 3602
Cao R, Thapa R, Kim H, et al. Promotion of oxygen reduction by a bio-inspired tethered iron phthalocyanine carbon nanotube-based catalyst. Nat Commun, 2013, 4: 2076
Koslowski UI, Abs-Wurmbach I, Fiechter S, et al. Nature of the catalytic centers of porphyrin-based electrocatalysts for the ORR: A correlation of kinetic current density with the site density of Fe-N4 centers. J Phys Chem C, 2008, 112: 15356–15366
Chen K, Liu K, An P, et al. Iron phthalocyanine with coordination induced electronic localization to boost oxygen reduction reaction. Nat Commun, 2020, 11: 4173
Pizarro A, Abarca G, Gutiérrez-Cerón C, et al. Building pyridinium molecular wires as axial ligands for tuning the electrocatalytic activity of iron phthalocyanines for the oxygen reduction reaction. ACS Catal, 2018, 8: 8406–8419
Li H, Pan Y, Wang Z, et al. Coordination engineering of cobalt phthalocyanine by functionalized carbon nanotube for efficient and highly stable carbon dioxide reduction at high current density. Nano Res, 2021, 15: 3056–3064
Kramer WW, McCrory CCL. Polymer coordination promotes selective CO2 reduction by cobalt phthalocyanine. Chem Sci, 2016, 7: 2506–2515
Liu Y, McCrory CCL. Modulating the mechanism of electrocatalytic CO2 reduction by cobalt phthalocyanine through polymer coordination and encapsulation. Nat Commun, 2019, 10: 1683
Rivera Cruz KE, Liu Y, Soucy TL, et al. Increasing the CO2 reduction activity of cobalt phthalocyanine by modulating the σ-donor strength of axially coordinating ligands. ACS Catal, 2021, 11: 13203–13216
Han N, Wang Y, Ma L, et al. Supported cobalt polyphthalocyanine for high-performance electrocatalytic CO2 reduction. Chem, 2017, 3: 652–664
Ko M, Mendecki L, Mirica KA. Conductive two-dimensional metal-organic frameworks as multifunctional materials. Chem Commun, 2018, 54: 7873–7891
Epstein A, Wildi BS. Electrical properties of poly-copper phthalocyanine. J Chem Phys, 1960, 32: 324–329
Sun C, Li Z, Yang J, et al. Two-dimensional closely packed amide polyphthalocyanine iron absorbed on Vulcan XC-72 as an efficient electrocatalyst for oxygen reduction reaction. Catal Today, 2020, 353: 279–286
Liu W, Hou Y, Pan H, et al. An ethynyl-linked Fe/Co heterometallic phthalocyanine conjugated polymer for the oxygen reduction reaction. J Mater Chem A, 2018, 6: 8349–8357
Jiao Y, Zheng Y, Jaroniec M, et al. Design of electrocatalysts for oxygenand hydrogen-involving energy conversion reactions. Chem Soc Rev, 2015, 44: 2060–2086
Riquelme J, Neira K, Marco JF, et al. Biomimicking vitamin B12. A Co phthalocyanine pyridine axial ligand coordinated catalyst for the oxygen reduction reaction. Electrochim Acta, 2018, 265: 547–555
Feaster JT, Shi C, Cave ER, et al. Understanding selectivity for the electrochemical reduction of carbon dioxide to formic acid and carbon monoxide on metal electrodes. ACS Catal, 2017, 7: 4822–4827
Varela AS, Sahraie NR, Steinberg J, et al. Metal-doped nitrogenated carbon as an efficient catalyst for direct CO2 electroreduction to CO and hydrocarbons. Angew Chem Int Ed, 2015, 54: 10758–10762
Bagger A, Ju W, Varela AS, et al. Single site porphyrine-like structures advantages over metals for selective electrochemical CO2 reduction. Catal Today, 2017, 288: 74–78
Hiratsuka K, Takahashi K, Sasaki H, et al. Electrocatalytic behavior of tetrasulfonated metal phthalocyanines in the reduction of carbon dioxide. Chem Lett, 1977, 6: 1137–1140
Meshitsuka S, Ichikawa M, Tamaru K. Electrocatalysis by metal phthalocyanines in the reduction of carbon dioxide. J Chem Soc Chem Commun, 1974, 158
Manassen J. Metal complexes of porphyrinlike compounds as heterogeneous catalysts. Catal Rev, 1974, 9: 223–243
Sakthinathan I, Mahendran M, Krishnan K, et al. Selenium tethered copper phthalocyanine hierarchical aggregates as electrochemical hydrogen evolution catalysts. Sustain Energy Fuels, 2021, 5: 3617–3631
Li C, Huang T, Huang Z, et al. A sulfonated cobalt phthalocyanine/ carbon nanotube hybrid as a bifunctional oxygen electrocatalyst. Dalton Trans, 2019, 48: 17258–17265
Aralekallu S, Sajjan VA, Palanna M, et al. Ni foam-supported azo linkage cobalt phthalocyanine as an efficient electrocatalyst for oxygen evolution reaction. J Power Sources, 2020, 449: 227516
Acknowledgements
This work was supported by the Research Fund of the National Research Foundation of Korea (NRF-2021R1A3B1077184, NRF-2021R1F1A1064057 and NRF-2021R1A4A5032876).
Author information
Authors and Affiliations
Contributions
Jeong DS and Yang J wrote and revised the manuscript; Shin HS proposed the topic and revised the manuscript. All authors contributed to the general discussion.
Corresponding authors
Additional information
Conflict of interest
The authors declare that they have no conflict of interest.
Da Sol Jeong received her BSc degree from Pukyeong National University in 2016, and MSc degree from Pusan National University in 2018. She is now a PhD candidate at the Department of Chemistry, Ulsan National Institute of Science and Technology (UNIST), Korea. She is currently working on the synthesis of 2D molecules and their applications for electrocatalysts.
Hyeon Suk Shin is a professor at the Department of Chemistry, UNIST, Korea. He received his PhD degree from Pohang University of Science and Technology (POSTECH) in 2002. After working as a postdoctoral fellow at the University of Cambridge, UK and subsequently as a research professor at POSTECH, he joined UNIST in 2008. His current research focuses on 2D materials, including graphene, h-BN, transition metal dichalcogenides, and their heterostructures, and their applications for electrocatalysts and (opto)electronic devices.
Jieun Yang is a professor at the Department of Chemistry and the Research Institute of Basic Sciences, Kyung Hee University. She received her PhD degree from the Interdisciplinary School of Green Energy, UNIST, in 2015. After working as a postdoctoral fellow at the University of Cambridge, UK, she joined Kyeong Hee University as a professor. Her current research focuses on transition metal dichalcogenides and their hybrids.
Rights and permissions
About this article
Cite this article
Jeong, D.S., Shin, H.S. & Yang, J. Influence of the molecular structure of metal-phthalocyanine on electrocatalytic reactions. Sci. China Mater. 65, 3324–3333 (2022). https://doi.org/10.1007/s40843-022-2131-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-022-2131-1