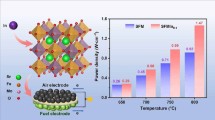
Abstract
Sc-doped Sr2Fe1.5Mo0.5O6−δ (SFMSc) was successfully synthesized by partially substituting Mo in Sr2Fe1.5Mo0.5O6−δ* (SFM) with Sc, resulting in a higher proton diffusion rate in the resultant SFMSc sample. Theoretical calculations showed that doping Sc into SFM lowered the oxygen vacancy formation energy, reduced the energy barrier for proton migration in the oxide, and increased the catalytic activity for oxygen reduction reaction. Next, a proton-conducting solid oxide fuel cell (H-SOFC) with a single-phase SFMSc cathode demonstrated significantly higher cell performance than that of cell based on an Sc-free SFM cathode, achieving 1258 mW cm−2 at 700°C. The performance also outperformed that of many other H-SOFCs based on single-phase cobalt-free cathodes. Furthermore, no trade-off between fuel cell performance and material stability was observed. The SFMSc material demonstrated good stability in both the CO2-containing atmosphere and the fuel cell application. The combination of high performance and outstanding stability suggests that SFMSc is an excellent cathode material for H-SOFCs.

摘要
本文利用Sc元素部分取代Sr2Fe1.5Mo0.5O6−δ (SFM) 中的Mo, 成功制备了具有高质子扩散速率的新型Sc掺杂SFM (SFMSc)材料. 理论计算表明, 将Sc掺杂到SFM中可以降低材料的氧空位形成能, 降低氧化物中质子迁移的能垒, 并提高材料氧还原反应的催化活性. 使用单相SFMSc阴极的质子导体固体氧化物燃料电池(H-SOFC)比使用不含Sc的SFM单相阴极电池具有更高的电池性能, 其在700°C时的性能达到1258 mW cm−2. 该性能也超过了许多其他使用单相无钴阴极的H-SOFC. 此外, 材料良好的电化学性能并没有以牺牲其稳定性为代价. SFMSc材料在含CO2 的气氛中以及在燃料电池工作条件下都表现出良好的稳定性. 高输出性能和良好的稳定性, 使SFMSc成为一种有潜力的高效的H-SOFC阴极材料.
Similar content being viewed by others
References
Zhang Y, Chen B, Guan D, et al. Thermal-expansion offset for high-performance fuel cell cathodes. Nature, 2021, 591: 246–251
Wachsman E, Ishihara T, Kilner J. Low-temperature solid-oxide fuel cells. MRS Bull, 2014, 39: 773–779
Chen K, Jiang SP. Surface segregation in solid oxide cell oxygen electrodes: Phenomena, mitigation strategies and electrochemical properties. Electrochem Energ Rev, 2020, 3: 730–765
Kilner JA, Burriel M. Materials for intermediate-temperature solid-oxide fuel cells. Annu Rev Mater Res, 2014, 44: 365–393
Li P, Yang W, Tian C, et al. Electrochemical performance of La2NiO4+δCe0.55La0.45O2−δ as a promising bifunctional oxygen electrode for reversible solid oxide cells. J Adv Ceram, 2021, 10: 328–337
Wang W, Tian Y, Liu Y, et al. Tailored Sr-Co-free perovskite oxide as an air electrode for high-performance reversible solid oxide cells. Sci China Mater, 2021, 64: 1621–1631
Li Y, Singh M, Zhuang Z, et al. Efficient reversible CO/CO2 conversion in solid oxide cells with a phase-transformed fuel electrode. Sci China Mater, 2021, 64: 1114–1126
Chen M, Chen D, Wang K, et al. Densification and electrical conducting behavior of BaZr0.9Y0.1O3−δ proton conducting ceramics with NiO additive. J Alloys Compd, 2019, 781: 857–865
Medvedev D, Murashkina A, Pikalova E, et al. BaCeO3: Materials development, properties and application Prog Mater Sci, 2014, 60: 72–129
Li J, Wang C, Wang X, et al. Sintering aids for proton-conducting oxides—A double-edged sword? A mini review. Electrochem Commun, 2020, 112: 106672
Dai H, Kou H, Wang H, et al. Electrochemical performance of protonic ceramic fuel cells with stable bazro3-based electrolyte: A mini-review. Electrochem Commun, 2018, 96: 11–15
Tarutin AP, Lyagaeva JG, Medvedev DA, et al. Recent advances in layered Ln2NiO4+δ nickelates: fundamentals and prospects of their applications in protonic ceramic fuel and electrolysis cells. J Mater Chem A, 2021, 9: 154–195
Chen M, Xie X, Guo J, et al. Space charge layer effect at the platinum anode/BaZr0.9Y0.1O3−δ electrolyte interface in proton ceramic fuel cells. J Mater Chem A, 2020, 8: 12566–12575
Bi L, Boulfrad S, Traversa E Steam electrolysis by solid oxide electrolysis cells (SOECs) with proton-conducting oxides. Chem Soc Rev, 2014, 43: 8255–8270
Fabbri E, Bi L, Pergolesi D, et al. Towards the next generation of solid oxide fuel cells operating below 600°C with chemically stable proton-conducting electrolytes. Adv Mater, 2012, 24: 195–208
Peng R, Wu T, Liu W, et al. Cathode processes and materials for solid oxide fuel cells with proton conductors as electrolytes. J Mater Chem, 2010, 20: 6218–6225
Lin Y, Ran R, Zhang C, et al. Performance of PrBaCo2O5+δ as a proton-conducting solid-oxide fuel cell cathode. J Phys Chem A, 2010, 114: 3764–3772
Guo Y, Lin Y, Ran R, et al. Zirconium doping effect on the performance of proton-conducting BaZryCe0.8−y Y0.2O3−δ (0.0≤y≤0.8) for fuel cell applications. J Power Sources, 2009, 193: 400–407
Kim J, Sengodan S, Kwon G, et al. Triple-conducting layered perovskites as cathode materials for proton-conducting solid oxide fuel cells. ChemSusChem, 2014, 7: 2811–2815
Choi S, Kucharczyk CJ, Liang Y, et al. Exceptional power density and stability at intermediate temperatures in protonic ceramic fuel cells. Nat Energy, 2018, 3: 202–210
Xu X, Xu Y, Ma J, et al. Tailoring electronic structure of perovskite cathode for proton-conducting solid oxide fuel cells with high performance. J Power Sources, 2021, 489: 229486
Wang N, Hinokuma S, Ina T, et al. Mixed proton-electron-oxide ion triple conducting manganite as an efficient cobalt-free cathode for protonic ceramic fuel cells. J Mater Chem A, 2020, 8: 11043–11055
Zhou C, Sunarso J, Song Y, et al. New reduced-temperature ceramic fuel cells with dual-ion conducting electrolyte and triple-conducting double perovskite cathode. J Mater Chem A, 2019, 7: 13265–13274
Xu X, Wang H, Fronzi M, et al. Tailoring cations in a perovskite cathode for proton-conducting solid oxide fuel cells with high performance. J Mater Chem A, 2019, 7: 20624–20632
Fabbri E, Pergolesi D, Traversa E. Materials challenges toward proton-conducting oxide fuel cells: A critical review. Chem Soc Rev, 2010, 39: 4355–4369
Bi L, Shafi SP, Da’as EH, et al. Tailoring the cathode-electrolyte interface with nanoparticles for boosting the solid oxide fuel cell performance of chemically stable proton-conducting electrolytes. Small, 2018, 14: 1801231
Song Y, Chen Y, Wang W, et al. Self-assembled triple-conducting nanocomposite as a superior protonic ceramic fuel cell cathode. Joule, 2019, 3: 2842–2853
Tong X, Xu Y, Tripkovic D, et al. Promotion of oxygen reduction and evolution by applying a nanoengineered hybrid catalyst on cobalt free electrodes for solid oxide cells. J Mater Chem A, 2020, 8: 9039–9048
Yuan R, He W, Zhang C, et al. Cobalt free SrFe0.95Nb0.05O3−δ cathode material for proton-conducting solid oxide fuel cells with BaZr0.1Ce0.7-Y0.2O3−δ electrolyte. Mater Lett, 2017, 200: 75–78
Muñoz-García AB, Pavone M, Ritzmann AM, et al. Oxide ion transport in Sr2Fe1.5Mo0.5O6−δ, a mixed ion-electron conductor: new insights from first principles modeling. Phys Chem Chem Phys, 2013, 15: 6250–6259
Liu Q, Bugaris DE, Xiao G, et al. Sr2Fe1.5Mo0.5O6−δ as a regenerative anode for solid oxide fuel cells J Power Sources, 2011, 196: 9148–9153
Meng X, Wang Y, Zhao Y, et al. In-situ exsolution of nanoparticles from Ni substituted Sr2Fe1.5Mo0.5O6 perovskite oxides with different Ni doping contents Electrochim Acta, 2020, 348: 136351
Yang Y, Wang Y, Yang Z, et al. Co-substituted Sr2Fe1.5Mo0.5O6−δ as anode materials for solid oxide fuel cells: Achieving high performance via nanoparticle exsolution J Power Sources, 2019, 438: 226989
Xu C, Zhen S, Ren R, et al. Cu-doped Sr2Fe1.5Mo0.5O6−δ as a highly active cathode for solid oxide electrolytic cells Chem Commun, 2019, 55: 8009–8012
Qiu P, Lin J, Lei L, et al. Evaluation of Cr-tolerance of the Sr2Fe1.5Mo0.5O6−δ cathode for solid oxide fuel cells. ACS Appl Energy Mater, 2019, 2: 7619–7627
Ren R, Wang Z, Meng X, et al. Tailoring the oxygen vacancy to achieve fast intrinsic proton transport in a perovskite cathode for protonic ceramic fuel cells ACS Appl Energy Mater, 2020, 3: 4914–4922
Da’as EH, Bi L, Boulfrad S, et al. Nanostructuring the electronic conducting La0.8Sr0.2MnO3−δ cathode for high-performance in proton-conducting solid oxide fuel cells below 600°C. Sci China Mater, 2018, 61: 57–64
Kresse G, Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set Phys Rev B, 1996, 54: 11169–11186
Muñoz-García AB, Tuccillo M, Pavone M Computational design of cobalt-free mixed proton-electron conductors for solid oxide electrochemical cells J Mater Chem A, 2017, 5: 11825–11833
Henkelman G, Uberuaga BP, Jónsson H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J Chem Phys, 2000, 113: 9901–9904
Liu W, Kou H, Wang X, et al. Improving the performance of the Ba0.5Sr0.5Co0.8Fe0.2O3 cathode for proton-conducting SOFCs by microwave sintering. Ceramics Int, 2019, 45: 20994–20998
Lu X, Yang X, Jia L, et al. First principles study on the oxygen reduction reaction of the La1−xSrxMnO3 coated Ba1−xSrxCo1−yFeyO3 cathode for solid oxide fuel cells Int J Hydrogen Energy, 2019, 44: 16359–16367
Zhang X, Pei C, Chang X, et al. FeO6 octahedral distortion activates lattice oxygen in perovskite ferrite for methane partial oxidation coupled with CO2 splitting. J Am Chem Soc, 2020, 142: 11540–11549
Xu Y, Xu X, Cao N, et al. Perovskite ceramic oxide as an efficient electrocatalyst for nitrogen fixation Int J Hydrogen Energy, 2021, 46: 10293–10302
Xu Y, Liu X, Cao N, et al. Defect engineering for electrocatalytic nitrogen reduction reaction at ambient conditions Sustain Mater Technologies, 2021, 27: e00229
Ji Q, Xu X, Liu X, et al. Improvement of the catalytic properties of porous lanthanum manganite for the oxygen reduction reaction by partial substitution of strontium for lanthanum Electrochem Commun, 2021, 124: 106964
Wu S, Xu X, Li X, et al. High-performance proton-conducting solid oxide fuel cells using the first-generation Sr-doped LaMnO3 cathode tailored with Zn ions Sci China Mater, 2022, 65: 675–682
Han D, Uemura S, Hiraiwa C, et al. Detrimental effect of sintering additives on conducting ceramics: yttrium-doped barium zirconate. ChemSusChem, 2018, 11: 4102–4113
Tao Z, Xu X, Bi L. Density functional theory calculations for cathode materials of proton-conducting solid oxide fuel cells: A mini-review. Electrochem Commun, 2021, 129: 107072
Kreuer KD. Proton-conducting oxides. Annu Rev Mater Res, 2003, 33: 333–359
Ji Q, Bi L, Zhang J, et al. The role of oxygen vacancies of ABO3 perovskite oxides in the oxygen reduction reaction. Energy Environ Sci, 2020, 13: 1408–1428
Tao Z, Bi L, Yan L, et al. A novel single phase cathode material for a proton-conducting SOFC. Electrochem Commun, 2009, 11: 688–690
Tao Z, Bi L, Zhu Z, et al. Novel cobalt-free cathode materials BaCexFe1−xO3−δ for proton-conducting solid oxide fuel cells. J Power Sources, 2009, 194: 801–804
Rao Y, Zhong S, He F, et al. Cobalt-doped BaZrO3: A single phase air electrode material for reversible solid oxide cells. Int J Hydrogen Energy, 2012, 37: 12522–12527
Zhang Y, Zhu A, Guo Y, et al. Electrochemical performance and effect of moisture on Ba0.5Sr0.5Sc0.175Nb0.025Co0.8O3−δ oxide as a promising electrode for proton-conducting solid oxide fuel cells. Appl Energy, 2019, 238: 344–350
Zhang Z, Wang J, Chen Y, et al. In situ formation ofa 3D core-shell and triple-conducting oxygen reduction reaction electrode for proton-conducting SOFCs J Power Sources, 2018, 385: 76–83
Shao L, Si F, Fu XZ, et al. Stable SrCo0.7Fe0.2Zr0.1O3−δ cathode material for proton conducting solid oxide fuel cell reactors Int J Hydrogen Energy, 2018, 43: 7511–7514
Ma J, Tao Z, Kou H, et al. Evaluating the effect of Pr-doping on the performance of strontium-doped lanthanum ferrite cathodes for protonic SOFCs Ceramics Int, 2020, 46: 4000–4005
He B, Zhang L, Zhang Y, et al. New insight into highly active cathode of proton conducting solid oxide fuel cells by oxygen ionic conductor modification J Power Sources, 2015, 287: 170–176
Bae K, Jang DY, Choi HJ, et al. Demonstrating the potential of yttrium-doped barium zirconate electrolyte for high-performance fuel cells Nat Commun, 2017, 8: 14553
Duan C, Tong J, Shang M, et al. Readily processed protonic ceramic fuel cells with high performance at low temperatures Science, 2015, 349: 1321–1326
Wang Z, Yang W, Shafi SP, et al. A high performance cathode for proton conducting solid oxide fuel cells J Mater Chem A, 2015, 3: 8405–8412
Yang S, Wen Y, Zhang J, et al. Electrochemical performance and stability of cobalt-free Ln1.2Sr0.8NiO4 (Ln=La and Pr) air electrodes for proton-conducting reversible solid oxide cells Electrochim Acta, 2018, 267: 269–277
Tang H, Jin Z, Wu Y, et al. Cobalt-free nanofiber cathodes for proton conducting solid oxide fuel cells. Electrochem Commun, 2019, 100: 108–112
Xia Y, Jin Z, Wang H, et al. A novel cobalt-free cathode with triple-conduction for proton-conducting solid oxide fuel cells with unprecedented performance. J Mater Chem A, 2019, 7: 16136–16148
Miao L, Hou J, Gong Z, et al. A high-performance cobalt-free Ruddlesden-Popper phase cathode La1.2Sr0.8Ni0.6Fe0.4O4+δ for low temperature proton-conducting solid oxide fuel cells. Int J Hydrogen Energy, 2019, 44: 7531–7537
Wang Q, Hou J, Fan Y, et al. Pr2BaNiMnO7−δ double-layered Ruddlesden-Popper perovskite oxides as efficient cathode electrocatalysts for low temperature proton conducting solid oxide fuel cells J Mater Chem A, 2020, 8: 7704–7712
Matsumoto H, Sakai T, Okuyama Y. Proton-conducting oxide and applications to hydrogen energy devices. Pure Appl Chem, 2013, 85: 427–435
Acknowledgements
This work was supported by the National Natural Science Foundation of China (51972183) and the Startup Funding for Talents at the University of South China.
Author information
Authors and Affiliations
Contributions
Author contributions Zhang L, Yin Y, and Bi L designed the study and analyzed the data. Zhang L, Yin Y, Xu Y, and Yu S performed the experiments. Bi L wrote the manuscript with other co-authors, and all authors discussed the results and provided their approval to the final version.
Corresponding author
Ethics declarations
Conflict of interest The authors declare that they have no conflict of interest.
Additional information
Supplementary information Supporting data are available in the online version of the paper.
Liling Zhang is a postgraduate student in Prof. Lei Bi’s group at the University of South China. Her research interests focus mostly on the development of new cathode materials for H-SOFCs and the exploration of the influence of microwave sintering on the characteristics of materials and the performance of H-SOFCs.
Yanru Yin was a research assistant in Prof. Lei Bi’s group at the University of South China after she received her master’s degree from Qingdao University. Her research interest is in tailoring the structure and properties of proton-conducting oxides, with the goal of understanding the charge carrier transport mechanism in proton-conducting oxides and then improving the performance of H-SOFCs.
Lei Bi is a full professor at the University of South China and leads a research group that studies H-SOFCs utilizing both first-principles calculations and experimental methodologies. His research interests include the development and optimization of essential materials for H-SOFCs, as well as innovative fuel cell fabrication technologies. Another area of study that he is interested in is the development of new sintering processes for H-SOFC fabrication.
Rights and permissions
About this article
Cite this article
Zhang, L., Yin, Y., Xu, Y. et al. Tailoring Sr2Fe1.5Mo0.5O6−δ with Sc as a new single-phase cathode for proton-conducting solid oxide fuel cells. Sci. China Mater. 65, 1485–1494 (2022). https://doi.org/10.1007/s40843-021-1935-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-021-1935-5