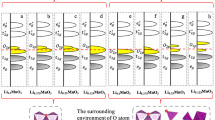
Abstract
The development of next-generation layered oxide cathodes for high-energy-density electrical vehicle Li-ion batteries (LIBs) is an urgent topic. The existing method is achieved by continuously increasing the Ni contents of Ni-based layered oxides, but it has been limited to LiNiO2. To break this limit and attain increased energy densities, a promising strategy, which involves the introduction of excess Li ions into transition metal (TM) layers to form Li-excess compounds Li2MO3 (M is a TM cation), has attracted enormous interest recently. However, another strategy, which has been neglected in recent years, involves the insertion of an extra layer of Li ions between the TM and original Li layers to form Li2MO2. In this study, typical reversible Li2NiO3 and 1T-Li2NiO2 were selected as two representative cathodes to break the limit of LiNiO2, thereby availing comprehensive comparison with LiNiO2 regarding their overall properties as cathodes from a theoretical perspective. Interestingly, dissimilar to the Ni3+/Ni4+ monoelectron cationic redox associated with LiNiO2, a polaronic anionic redox reaction occurs in Li2NiO3, while a reversible Ni2+/Ni4+ double-electron redox reaction accompanied by insulator-metal transition occurs in Li2NiO2. Owing to this double-electron cationic activity, Li2NiO2 exhibits absolute advantages over the other two materials (LiNiO2 and Li2NiO3) as cathodes for LIBs in terms of the capacity, energy density, electronic conductivity, and thermal stability, thus rendering it the most promising candidate for next-generation layered oxide cathodes with high energy densities to break the limit of LiNiO2.

摘要
开发新一代层状氧化物阴极, 是发展高能量密度电动汽车锂离子电池迫切关注的问题. 目前有一种方法是不断提高镍基层状氧化物中的镍含量, 但这一方法的极限是LiNiO2. 为了突破这一极限, 获得更高的能量密度, 近年来备受关注的一种方法是在过渡金属层中引入过量的锂离子, 形成Li2MO3 (M是过渡金属阳离子). 然而, 还有一种一直被忽视的方法是在过渡金属层和原始Li层之间插入一层额外的Li离子, 形成Li2MO2. 本研究中, 我们选择了典型的Li2NiO3和1T-Li2NiO2作为代表, 从理论角度综合比较了Li2NiO3、 1T-Li2NiO2和LiNiO2的各项电化学性能. 我们发现, 不同于LiNiO2中发生的Ni3+/Ni4+单电子阳离子氧化还原, 在Li2NiO3中存在着伴有极化子的阴离子氧化还原. 而在Li2NiO2中, 则发生了伴有绝缘体到金属转变的Ni2+/Ni4+双电子氧化还原. 在这三种材料中, 由于Li2NiO2具有双电子氧化还原活性, 其在容量、 能量密度、 电导率和热稳定性等方面都表现优异, 是最有希望突破LiNiO2极限的下一代层状氧化物阴极材料. 虽然Li2NiO2具有脱锂过程中的体积变化较大的缺点, 但我们提出了两种可能的解决方法: 在Li层中掺杂Na和在TM层中掺杂Mo, 都获得了不错的效果. 这一工作为如何突破Li-NiO2的能量密度极限, 开发下一代具有高能量密度的层状氧化物阴极提供了新的思路.
Similar content being viewed by others
References
Kuo LY, Guillon O, Kaghazchi P. On the origin of non-monotonic variation of the lattice parameters of LiNi1/3Co1/3Mn1/3O2 with lithiation/delithiation: a first-principles study. J Mater Chem A, 2020, 8: 13832–13841
Choi J, Manthiram A. Role of chemical and structural stabilities on the electrochemical properties of layered LiNi1/3Mn1/3Co1/3O2 cathodes. J Electrochem Soc, 2005, 152: A1714
Lee E, Persson KA. Structural and chemical evolution of the layered Li-excess LixMnO3 as a function of Li content from first-principles calculations. Adv Energy Mater, 2014, 4: 1400498
Marusczyk A, Albina JM, Hammerschmidt T, et al. Oxygen activity and peroxide formation as charge compensation mechanisms in Li2MnO3. J Mater Chem A, 2017, 5: 15183–15190
Jiao C, Xu G, Wang M, et al. Improving electrochemical performance of Li-rich cathode materials via adjusting the components. Solid State Ion, 2021, 369: 115722
Ramos-Sanchez G, Romero-Ibarra IC, Vazquez-Arenas J, et al. Controlling Li2CuO2 single phase transition to preserve cathode capacity and cyclability in Li-ion batteries. Solid State Ion, 2017, 303: 89–96
Dahn J. Structure and electrochemistry of Li1±yNiO2 and a new Li2NiO2 phase with the Ni(OH)2 structure. Solid State Ion, 1990, 44: 87–97
Kang K, Chen CH, Hwang BJ, et al. Synthesis, electrochemical properties, and phase stability of Li2NiO2 with the Immm structure. Chem Mater, 2004, 16: 2685–2690
Lin CJ, Zheng F, Zhu ZZ. Electronic structures and Li diffusion in cathode material Li2FeO2 of Li-ion batteries. Acta Phys Sin, 2019, 68: 157201
Wu Z, Ji S, Zheng J, et al. Prelithiation activates Li(Ni0.5Mn0.3Co0.2)O2 for high capacity and excellent cycling stability Nano Lett, 2015, 15: 5590–5596
Love CT, Dmowski W, Johannes MD, et al. Structural originations of irreversible capacity loss from highly lithiated copper oxides J Solid State Chem, 2011, 184: 2412–2419
Aguilar-Eseiza N, Ramos-Sánchez G, González F, et al. High voltage-improved reversible capacity in Ni+2/+3 modified copper-based cathodes for lithium ion batteries Electrochem Commun, 2018, 96: 32–36
Martínez-Cruz MA, Yañez-Aulestia A, Ramos-Sánchez G, et al. Unraveling the effects on lithium-ion cathode performance by cation doping M-Li2CuO2 solid solution samples (M = Mn, Fe and Ni). Dalton Trans, 2020, 49: 4549–4558
Arachi Y, Ide T, Nakagawa T, et al. Crystal structures and electrochemical properties of Ni-substituted Li2CuO2 as a cathode material ECS Trans, 2013, 50: 143–151
Kordatos A, Kuganathan N, Kelaidis N, et al. Defects and lithium migration in Li2CuO2 Sci Rep, 2018, 8: 7
Ruther RE, Samuthira Pandian A, Yan P, et al. Structural transformations in high-capacity Li2Cu0.5Ni0.5O2 cathodes. Chem Mater, 2017, 29: 2997–3005
Lee H, Chang SK, Goh EY, et al. Li2NiO2 as a novel cathode additive for overdischarge protection of Li-ion batteries Chem Mater, 2008, 20: 5–7
Park H, Yoon T, Kim YU, et al. Li2NiO2 as a sacrificing positive additive for lithium-ion batteries Electrochim Acta, 2013, 108: 591–595
Johnson CS, Kim JS, Kropf AJ, et al. Structural characterization of layered LixNi0.5Mn0.5O2 (0 < x ≤ 2) oxide electrodes for Li batteries. Chem Mater, 2003, 15: 2313–2322
Perdew JP, Chevary JA, Vosko SH, et al. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation Phys Rev B, 1992, 46: 6671–6687
Ong SP, Hautier G., Kocher M, et al. The materials project (https://www.Materialsproject.Org/)
Bianchini M, Roca-Ayats M, Hartmann P, et al. There and back again—The journey of LiNiO2 as a cathode active material. Angew Chem Int Ed, 2019, 58: 10434–10458
Dyer LD, BorieJr. BS, Smith GP. Alkali metal-nickel oxides of the type MNiO2. J Am Chem Soc, 1954, 76: 1499–1503
Migeon HN, Zanne M, Gleitzer C, et al. The Li2O-NiO-O2 system at 670°C and the consequences of non-stoichiometry on the magnetic properties of the LixNi1−xO1±y phases. J Mater Sci, 1978, 13: 461–466
Shinova E, Zhecheva E, Stoyanova R, et al. High-pressure synthesis of solid solutions between trigonal LiNiO2 and monoclinic Li[Li1/3Ni2/3] O2. J Solid State Chem, 2005, 178: 1661–1669
Billerey D, Terrier C, Mainard R, et al. Magnetic-structure of NiCl2 at 4.2 K by neutron-diffraction. Comptes Rendus Hebdomadaires Des Seances De L Acad Des Sci B, 1977, 284: 495–498
Momeni M, Yousefi Mashhour H, Kalantarian MM. New approaches to consider electrical properties, band gaps and rate capability of same-structured cathode materials using density of states diagrams: Layered oxides as a case study. J Alloys Compd, 2019, 787: 738–743
Molenda J. Structural, electrical and electrochemical properties of Li-NiO2. Solid State Ion, 2002, 146: 73–79
Zheng J, Ye Y, Pan F. ‘Structure units’ as material genes in cathode materials for lithium-ion batteries. Natl Sci Rev, 2020, 7: 242–245
Wang ZQ, Wu MS, Xu B, et al. Improving the electrical conductivity and structural stability of the Li2MnO3 cathode via P doping. J Alloys Compd, 2016, 658: 818–823
Ye YK, Hu ZX, Liu JH, et al. Research progress of theoretical studies on polarons in cathode materials of lithium-ion batteries. Acta Phys-Chim Sin, 2021, 37: 2011003
Bianchini M, Schiele A, Schweidler S, et al. From LiNiO2 to Li2NiO3: Synthesis, structures and electrochemical mechanisms in Li-rich nickel oxides. Chem Mater, 2020, 32: 9211–9227
Marianetti CA, Kotliar G, Ceder G. A first-order Mott transition in LixCoO2. Nat Mater, 2004, 3: 627–631
Jung R, Metzger M, Maglia F, et al. Oxygen release and its effect on the cycling stability of LiNixMnyCozO2 (NMC) cathode materials for Li-ion batteries. J Electrochem Soc, 2017, 164: A1361–A1377
Li N, Sallis S, Papp JK, et al. Unraveling the cationic and anionic redox reactions in a conventional layered oxide cathode. ACS Energy Lett, 2019, 4: 2836–2842
Sathiya M, Rousse G, Ramesha K, et al. Reversible anionic redox chemistry in high-capacity layered-oxide electrodes. Nat Mater, 2013, 12: 827–835
Zheng J, Teng G, Yang J, et al. Mechanism of exact transition between cationic and anionic redox activities in cathode material Li2FeSiO4. J Phys Chem Lett, 2018, 9: 6262–6268
Strehle B, Kleiner K, Jung R, et al. The role of oxygen release from Li-and Mn-rich layered oxides during the first cycles investigated by on-line electrochemical mass spectrometry. J Electrochem Soc, 2017, 164: A400–A406
Yan HJ, Li B, Jiang N, et al. First-principles study: The structural stability and sulfur anion redox of Li1−xNio2−ySy. Acta Phys-Chim Sin, 2017, 33: 1781–1788
Sun G, Yu FD, Que LF, et al. Local electronic structure modulation enhances operating voltage in Li-rich cathodes. Nano Energy, 2019, 66: 104102
Yao Z, Kim S, He J, et al. Interplay of cation and anion redox in Li4Mn2O5 cathode material and prediction of improved Li4(Mn,M)2O5 electrodes for Li-ion batteries. Sci Adv, 2018, 4: eaao6754
Acknowledgements
This work was financially supported by the starting fund of Peking University, Shenzhen Graduate School and Fujian Science & Technology Innovation Laboratory for Energy Devices of China (21C-LAB).
Author information
Authors and Affiliations
Contributions
Author contributions Jia Y, Pan F and Zheng J conceived the idea and designed the project. Jia Y performed all the calculations. Ye Y wrote the programs to process the data. Liu J, Zheng S, Lin W, Wang Z, and Li S analyzed the results and participated in the discussion of mechanism. Jia Y and Zheng J wrote the manuscript and all authors edited the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest The authors declare that they have no conflict of interest.
Additional information
Supplementary information Details for the calculations and supporting data are available in the online version of the paper.
Yining Jia is a master student at the School of Advanced Materials, Peking University, Shenzhen Graduate School. Her research focuses on computational materials and energy materials.
Feng Pan is Chair-Professor, Founding Dean of the School of Advanced Materials, Peking University, Shenzhen Graduate School. He received his PhD degree from the Department of P&A Chemistry, University of Strathclyde, Glasgow, U.K., receiving the “Patrick D. Ritchie Prize” for the best PhD in 1994. Prof. Pan has been engaged in fundamental research and product development of novel optoelectronic and energy storage materials and devices.
Jiaxin Zheng received his PhD degree in condensed physics from Peking University in 2013. He is currently working at the School of Advanced Materials, Peking University as an Associate Professor. His research interests include computational materials and energy materials.
Supplementary information
Rights and permissions
About this article
Cite this article
Jia, Y., Ye, Y., Liu, J. et al. Breaking the energy density limit of LiNiO2: Li2NiO3 or Li2NiO2?. Sci. China Mater. 65, 913–919 (2022). https://doi.org/10.1007/s40843-021-1827-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-021-1827-x