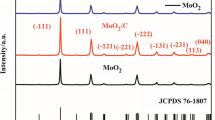
Abstract
Lithium nickel manganese oxide spinel (LiNi0.5Mn1.5O4, LNMO) has attracted much attention as the cathode material for rechargeable lithium-ion batteries due to its high energy density and low cost. However, the short cycle life and poor high-rate capability hinder its commercialization. In this study, we synthesized hollow spherical LNMO built from polyhedral particles. The LNMO hollow structure guarantees sufficient contact with electrolyte and rapid diffusion of lithium ions. To enhance the conductivity, we use carbon nanotubes (CNTs) to modify the surface of the cathode. After CNT modification, the LNMO hollow structure manifests outstanding cycling stability and high-rate capability. It delivers a discharge capacity of 127 mA h g−1 at 5 C, maintaining 104 mA h g−1 after 500 cycles. Even at a high rate of 20 C, a capacity of 121 mA h g−1 can be obtained. The excellent electrochemical performance is ascribed to the unique structure and the enhanced conductivity through CNT modification. It is demonstrated that the CNT-modified hollow spherical LNMO is a promising cathode for lithium ion batteries.
摘要
本文通过调节烧结温度设计构筑了一种纳米多面体颗粒堆积的中空球状LiNi0.5Mn1.5O4材料, 并进一步通过碳纳米管(CNT)的改性来提高材料的循环性能和高倍率性能. 纳米中空结构不仅减少了锂离子的扩散路径, 也保证了电解液和正极材料的充分接触, 三维网状CNT的 改性提高了材料的电子导电率, 从而明显改善了材料的循环和高倍率性能. 最终得到的LNMO-850/CNT材料在5 C的电流密度下初始容量为 127 mA h g−1, 500次循环后容量保持在104 mA h g−1. 而在20 C的高电流密度下容量仍达到121 mA h g−1, 体现了材料优异的循环和高倍率性能.
Similar content being viewed by others
References
Yang L, Ravdal B, Lucht BL. Electrolyte reactions with the surface of high voltage LiNi0.5Mn1.5O4 cathodes for lithium-ion batteries. Electrochem Solid State Lett, 2010, 13: A95–A97
Santhanam R, Rambabu B. Research progress in high voltage spinel LiNi0.5Mn1.5O4 material. J Power Sources, 2010, 195: 5442–5451
Ma X, Kang B, Ceder G. High rate micron-sized ordered Li-Ni0.5Mn1.5O4. J Electrochem Soc, 2010, 157: A925
Wang Y, Yang G, Yang Z, et al. High power and capacity of LiNi0.5Mn1.5O4 thin films cathodes prepared by pulsed laser deposition. Electrochim Acta, 2013, 102: 416–422
Xiao J, Chen X, Sushko PV, et al. High-performance LiNi0.5Mn1.5O4 spinel controlled by Mn3+ concentration and site disorder. Adv Mater, 2012, 24: 2109–2116
Lou XW, Archer LA, Yang Z. Hollow micro/nanostructures: synthesis and applications. Adv Mater, 2008, 20: 3987–4019
Yang J, Zhang X, Zhu Z, Cheng F, Chen J. Ordered spinel LiNi0.5Mn1.5O4 nanorods for high-rate lithium-ion batteries. J Electroanal Chem, 2013, 688: 113–117
Zhang X, Cheng F, Yang J, Chen J. LiNi0.5Mn1.5O4 porous nanorods as high-rate and long-life cathodes for Li-ion batteries. Nano Lett, 2013, 13: 2822–2825
Zhou L, Zhao D, Lou XD. LiNi0.5Mn1.5O4 hollow structures as high-performance cathodes for lithium-ion batteries. Angew Chem Int Ed, 2012, 51: 239–241
Jo M, Lee YK, Kim KM, Cho J. Nanoparticle-nanorod core-shell LiNi0.5Mn1.5O4 spinel cathodes with high energy density for Li-ion batteries. J Electrochem Soc, 2010, 157: A841–A845
Zhu X, Li X, Zhu Y, et al. Porous LiNi0.5Mn1.5O4 microspheres with different pore conditions: preparation and application as cathode materials for lithium-ion batteries. J Power Sources, 2014, 261: 93–100
Luo H, Nie P, She L, et al. Synthesis of LiNi0.5Mn1.5O4 hollow microspheres and their lithium-storage properties. ChemElectroChem, 2015, 2: 127–133
Li J, Xiong Y, Liu Y, Ju Z, Qian Y. Uniform LiNi1/3Co1/3Mn1/3O2 hollow microspheres: designed synthesis, topotactical structural transformation and their enhanced electrochemical performance. Nano Energy, 2013, 2: 1249–1260
Feng XY, Shen C, Fang X, Chen CH. Synthesis of LiNi0.5Mn1.5O4 by solid-state reaction with improved electrochemical performance. J Alloy Compd, 2011, 509: 3623–3626
Yang T, Zhang N, Lang Y, Sun K. Enhanced rate performance of carbon-coated LiNi0.5Mn1.5O4 cathode material for lithium ion batteries. Electrochim Acta, 2011, 56: 4058–4064
Ban C, Li Z, Wu Z, et al. Extremely durable high-rate capability of a LiNi0.4Mn0.4Co0.2O2 cathode enabled with single-walled carbon nanotubes. Adv Energy Mater, 2011, 1: 58–62
Wu ZZ, Han XG, Zheng JX, et al. Depolarized and fully active cathode based on Li(Ni0.5Co0.2Mn0.3)O2 embedded in carbon nanotube network for advanced batteries. Nano Lett, 2014, 14: 4700–4706
Thang VL, My LPL, Man VT, et al. Fabrication of cathode materials based on LiMn2O4/CNT and LiNi0.5Mn1.5O4/CNT nanocomposites for lithium-ion batteries application. Mater Res, 2015, 18: 1044–1052
Ohzuku T, Takeda S, Iwanaga M. Solid-state redox potentials for Li[Me1/2Mn3/2]O4 (Me: 3d-transition metal) having spinelframework structures: a series of 5 volt materials for advanced lithium-ion batteries. J Power Sources, 1999, 81–82: 90–94
Xu HY, Xie S, Ding N, et al. Improvement of electrochemical properties of LiNi0.5Mn1.5O4 spinel prepared by radiated polymer gel method. Electrochim Acta, 2006, 51: 4352–4357
Kim JH, Myung ST, Yoon CS, Kang SG, Sun YK. Comparative study of LiNi0.5Mn1.5O4-d and LiNi0.5Mn1.5O4 cathodes having two crystallographic structures: Fd3–m and P4332. Chem Mater, 2004, 16: 906–914
Amdouni N, Zaghib K, Gendron F, Mauger A, Julien CM. Structure and insertion properties of disordered and ordered LiNi0.5Mn1.5O4 spinels prepared by wet chemistry. Ionics, 2006, 12: 117–126
Ivanova S, Zhecheva E, Stoyanova R, et al. High-voltage LiNi0.5Mn1.5O4 spinel: cationic order and particle size distribution. J Phys Chem C, 2011, 11: 25170–25182
Kunduraci M, Amatucci GG. Synthesis and characterization of nanostructured 4.7 V Lix Ni0.5Mn1.5O4 spinels for high-power lithium- ion batteries. J Electrochem Soc, 2006, 153: A1345–A1352
Ariyoshi K, Iwakoshi Y, Nakayama N, Ohzuku T. Topotactic twophase reactions of Li[Ni1/2Mn3/2]O4 (P4332) in nonaqueous lithium cells. J Electrochem Soc, 2004, 151: A296–A303
Kunduraci M, Al-Sharab JF, Amatucci GG. High-power nanostructured LiMn2-x NixO4 high-voltage lithium-ion battery electrode materials: electrochemical impact of electronic conductivity and morphology. Chem Mater, 2006, 18: 3585–3592
Zhu Z, Yan H, Zhang D, Li W, Lu Q. Preparation of 4.7 V cathode material LiNi0.5Mn1.5O4 by an oxalic acid-pretreated solid-state method for lithium-ion secondary battery. J Power Sources, 2013, 224: 13–19
Lou XW, Lynden AA, Yang ZC. Hollow micro/nanostructures: synthesis and applications. Adv Mater, 2008, 20: 3987–4019
Jung SC, Young JH, Yun CK. Design and synthesis of bubble-nanorod- structured Fe2O3-carbon nanofibers as advanced anode material for Li-ion batteries. ACS Nano, 2015, 9: 4026–4035
Deng Y, Zhou Y, Shi Z, Zhou X, Quan X. Porous LiMn2O4 microspheres as durable high power cathode materials for lithium ion batteries. J Mater Chem A, 2013, A1: 8170–8177
Liu J, Hou M, Yi J, et al. Improving the electrochemical performance of layered lithium-rich transition-metal oxides by controlling the structural defects. Energy Environ Sci, 2014, 7: 705–714
Fang X, Ge MY, Rong JP, Zhou CW. Free-standing LiNi0.5Mn1.5O4/ carbon nanofiber network film as lightweight and high-power cathode for lithium ion batteries. ACS Nano, 2014, 8: 4876–4882
Yoon T, Park S, Mun J, et al. Failure mechanisms of LiNi0.5Mn1.5O4 electrode at elevated temperature. J Power Sources, 2012, 215: 312–316
Sun Y, Yang Y, Zhan H, Shao H, Zhou Y. Synthesis of high power type LiNi0.5Mn1.5O4 by optimizing its preparation conditions. J Power Sources, 2010, 195: 4322–4326
Lee ES, Nam KW, Hu E, Manthiram A. Influence of cation ordering and lattice distortion on the charge-discharge behavior of LiNi0.5Mn1.5O4 spinel between 5.0 and 2.0 V. Chem Mater, 2012, 24: 3610–3620
Cabana J, Casas-Cabanas M, Omenya FO, et al. Composition-structure relationships in the Li-ion battery electrode material LiNi0.5Mn1.5O4. Chem Mater, 2012, 24: 2952–2964
Lv YZ, Jin YZ, Xue Y, et al. Electrochemical properties of high-voltage LiNi0.5Mn1.5O4 synthesized by a solid-state method. RSC Adv, 2014, 4: 26022
Cheng JL, Li XH, Wang ZX, et al. Hydrothermal synthesis of LiNi0.5Mn1.5O4 sphere and its performance as high-voltage cathode material for lithium ion batteries. Ceram Int, 2016, 42: 3715–3719
Kang HB, Myung ST, Amine K, Lee SM, Sun YK. Electrochemical properties of high-voltage LiNi0.5Mn1.5O4 synthesized by a solid- state method. J Power Sources, 2010, 195: 2023–2028
Wu H M, Belharouak I, Abouimrane A, Sun YK, Amine K. Surface modification of LiNi0.5Mn1.5O4 by ZrP2O7 and ZrO2 for lithium-ion batteries. J Power Sources, 2010, 195: 2909–2913
Author information
Authors and Affiliations
Corresponding author
Additional information
Luoluo Wang received her bachelor degree from Southwest Jiaotong University in 2013. She is currently a graduate student at State key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology. Her research interest focuses on nano energy cathode materials.
Liqiang Mai received his PhD degree from Wuhan University of Technology in 2004 and then worked as a postdoctoral researcher at Georgia Institute of Technology in the group of Prof. Zhonglin Wang from 2006 to 2007. He conducted nanowire based nanodevices and battery research as advanced research scholar in the group of Prof. Charles M Lieber at Harvard University from 2008 to 2011. He was the winner of National Natural Sceince Fund for Distinguished Young Scholars in 2014. His current research field is nano energy materials and micro/nano devices. So far, he has published more than 130 papers tagged by SCI in leading journals.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Wang, L., Hu, Z., Zhao, K. et al. Hollow spherical LiNi0.5Mn1.5O4 built from polyhedra with high-rate performance via carbon nanotube modification. Sci. China Mater. 59, 95–103 (2016). https://doi.org/10.1007/s40843-016-0120-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-016-0120-3