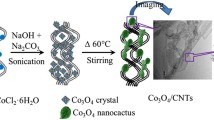
Abstract
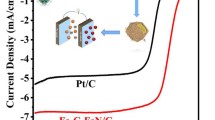
De veloping transition metal oxides/carbon substrate hybrids as highly promising non-precious metal oxygen reduction reaction (ORR) electrocatalysts is crucial to replace the scarce platinum and solve the world-wide energy predicament. In this work, γ-Fe2O3/N-carbon nanotubes (N-CNTs) and α-Fe2O3/N-CNTs nanocatalysts were successfully synthesized by simultaneous formation of crystal configuration of Fe2O3 and the doping of nitrogen on CNTs. α-Fe2O3/N-CNTs catalysts exhibited superior ORR electrocatalytic activity with lower onset and peak potential of -0.21 and -0.27 V, and possessed a more efficient four-electron-dominant ORR process compared with γ-Fe2O3/N-CNTs, N-CNTs and CNTs. The crystal distortions on octahedral α-Fe2O3 held great potential for displacement of either iron or other ions, serving as the active sites and contributing to its better ORR catalytic ability than the vacancies integrated in γ-Fe2O3/N-CNTs. Both the two nanocatalysts possessed superior methanol tolerance and long-term stabili ty of ORR compared with Pt/C, indicating great potential for their practical utilization in fuel cells.
中文摘要
本论文采用空气煅烧与氮气/氨气退火两步法制备了α-Fe2O3/N-CNTs和γ-Fe2O3/N-CNTs高效氧气还原反应催化剂. X射线 衍射与X射线光电子能谱等结果显示: 球状的α-Fe2O3与立方体状的γ-Fe2O3较好地分散在氮掺杂的碳纳米管上; 不同的退火温度造成 γ-Fe2O3/N-CNTs中氮的掺杂量约为1.06%, 而α-Fe2O3/N-CNTs中氮掺杂量约为1.94%. 从拉曼光谱结果发现, α-Fe2O3/N-CNTs的I D/I G值 (1.26)大于γ-Fe2O3/N-CNTs的I D/I G值(1.18), 说明α-Fe2O3/N-CNTs表面可因较大的碳缺陷程度而产生更多的氧还原活性电位. 电化学性 能表征结果再次印证: 相比较于γ-Fe2O3/N-CNTs, N-CNTs和CNTs, α-Fe2O3/N-CNTs具有更低的氧还原起始电位(−0.21 V)和峰值电位 (−0.27 V). 在碱性条件下, 氧气在α-Fe2O3/N-CNTs表面更易发生接近4电子的还原反应. 另外, 与Pt/C相比, α-Fe2O3/N-CNTs和γ-Fe2O3/ N-CNTs皆具有较好的催化耐久性与稳定性, 进一步显示了二者在清洁能源电池领域的应用价值与潜力.
Similar content being viewed by others
References
Yang Z, Zhou X, Nie H, Yao Z, Huang S. Facile construction of manganese oxide doped carb on nanotube catalysts with high activity for oxygen reduction reaction and investigations into the origin of their activity enhancement. ACS Appl Mater Interfaces, 2011, 3: 2601–2606
Dai L, Xue Y, Qu L, Choi HJ, Baek JB. Metal-free catalysts for oxygen reduction reaction. Chem Rev, 2015, 115: 4823–4892
Sun M, Liu H, Liu Y, Qu J, Li J. Graphene-based transition metal oxide nanocomposites for the oxygen reduction reaction. Nanoscale, 2015, 7: 1250–1269
Liu M, Zhang R, Chen W. Graphene-supported nanoelectrocatalysts for fuel cells: synthesis, properties, and applications. Chem Rev, 2014, 114: 5117–5160
Qian F, Wang H, Ling Y, et al. Photoenhanced electrochemical interaction between shewanella and a hematite nanowire photoanode. Nano Letters, 2014, 14: 3688–3693
Sun Z, Yuan H, Liu Z, Han B, Zhang X. A highly efficient chemical sensor material for H2S: α-Fe2O3 nanotubes fabricated using carbon nanotube templates. Adv Mater, 2005, 17: 2993–2997
Kay A, Cesar I, Grätzel M. New benchmark for water photooxidation by nanostructured α-Fe2O3 films. J Am Chem Soc, 2006, 128: 15714–15721
Sun M, Dong Y, Zhang G, Qu J, Li J. α-Fe2O3 spherical nanocrystals supported on CNTs as efficient non-noble electrocatalysts for the oxygen reduction reaction. J Mater Chem A, 2014, 2: 13635–13640
Zhou W, Lin L, Wang W, et al. Hierarchial mesoporous hematite with “electron-transport channels” and its improved performances in photocatalysis and lithium ion batteries. J Phys Chem C, 2011, 115: 7126–7133
Wu C, Yin P, Zhu X, OuYang C, Xie Y. Synthesis of hematite (α-Fe2O3) nanorods: diameter-size and s hape effects on their applications in magnetism, lithium ion battery, and gas sensors. J Phys Chem B, 2006, 110: 17806–17812
Yun H, Liu X, Paik T, et al. Size- and composition-dependent radio frequency magnetic permeability of iron oxide nanocrystals. ACS Nano, 2014, 8: 12323–12337
Zhao N, Ma W, Cui Z, et al. Polyhedral maghemite nanocrystals prepared by a flame synthetic method: p reparations, characterizations, and catalytic properties. ACS Nano, 2009, 3: 1775–1780
Li N, Li K, Wang S, et al. Gold embedded maghemite hybrid nanowires and their gas sensing properties. ACS Appl Mater Interfaces, 2015, 7: 10534–10540
Wu Y, Zhu P, Reddy M, Chowdari B, Ramakrishna S. Maghemite nanoparticles on electrospun CNFs template as prospective lithium-ion battery anode. ACS Appl Mater Interfaces, 2014, 6: 1951–1958
Morin G, Ona-Nguema G, Wang Y, et al. Extended X-ray absorption fine structure analysis of arsenite a nd arsenate adsorption on maghemite. Environ Sci Technol, 2008, 42: 2361–2366
Wen Z, Li J. Hierarchically structured carbon nanocomposites as electrode materials for electrochemic al energy storage, conversion and biosensor systems. J Mater Chem, 2009, 19: 8707–8713
Chen R, Yan J, Liu Y, Li J. Three-dimensional nitrogen-doped graphene/MnO nanoparticle hybrids as a high-performance catalyst for oxygen reduction reaction. J Phys Chem C, 2015, 119: 8032–8037
Jing L, Qu Y, Su H, Yao C, Fu H. Synthesis of high-activity TiO2-based photocatalysts by compounding a small amount of porous nanosized LaFeO3 and the activity-enhanced mechanisms. J Phys Chem C, 2011, 115: 12375–12380
Dong Y, Wu Y, Liu M, Li J. Electrocatalysis on shape-controlled titanium nitride nanocrystals for the oxygen reduction reaction. ChemSusChem, 2013, 6: 2016–2021
Dong J, Qu X, Wang L, Wang T. Electrochemical behavior of nitrogen-doped carbon nanotube modifi ed electrodes. Acta Chim Sinica, 2007, 21: 2405–2410
Rao C, Cabrera C, Ishikawa Y. In search of the active site in nitrogen-doped carbon nanotube electrod es for the oxygen reduction reaction. J Phys Chem Lett, 2010, 1: 2622–2627
Eder D. Carbon nanotube-inorganic hybrids. Chem Rev, 2010, 110: 1348–1385
Kim D, Li O, Saito N. Enhan cement of ORR catalytic activity by multiple heteroatom-doped carbon materials. Phys Chem Chem Phys, 2015, 17: 407–413
Chen W, Pan X, Bao X. Tuning of redox properties of iron and iron oxides via encapsulation within car bon nanotubes. J Am Chem Soc, 2007, 129: 7421–7426
Wang L, Yin J, Zhao L, et al. Ion-exchanged route synthesis of Fe2NN-doped graphitic nanocarbons com posite as advanced oxygen reduction electrocatalyst. Chem Commun, 2013, 49: 3022–3024
Chung H, Won J, Zelenay P. Active and stable carbon nanotube/nanoparticle composite electrocatalyst for oxygen reduction. Nat Commun, 2013, 4: 1922–1927
Ci S, Cai P, Wen Z, Li J. Graphene-based electrode materials for microbial fuelcells. Sci China Mater, 2015, 58: 496–509
Wang Y, Shao Y, Matson D, Li J, Lin Y. Nitrogen-doped graphene and its application in electrochemical biosensing. ACS Nano, 2010, 4: 1790–1798
Zabet-Khosousi A, Dhirani A. Charge transport in nanoparticle assemblies. Chem Rev, 2008, 108: 4072–4124
Xing T, Zheng Y, Li L, et al. Observation of active sites for oxygen reduction reaction on nitrogen-doped multilayer graphene. ACS Nano, 2014, 8: 6856–6862
Silva R, Voiry D, Chhowalla M, Asefa T. Efficient metal-free electrocatalysts for oxygen reduction: polyaniline-derived N- and O-doped mesoporous carbons. J Am Chem Soc, 2013, 135: 7823–7826
Author information
Authors and Affiliations
Corresponding author
Additional information
Jinghong Li is currently a Cheung Kong Professor at the Department of Chemistry at Tsinghua University, China. He received his BSc degree from the University of Science and Technology of China in 1991, and PhD degree from Changchun Institute of Applied Chemistry, Chinese Academy of Sciences in 1996. He spent several years at the University of Illinois at Urbana-Champaign, University of California at Santa Barbara, Clemson University, and Evonyx Inc., New York. He returned to Changchun in May 2001 and then joined the faculty of Tsinghua University in July 2004. His current research interests include electroanalytical chemistry, bio-electrochemistry and sensors, physical electrochemistry and interfacial electrochemistry, electrochemical materials science and nanoscopic electrochemistry, fundamental aspects of energy conversion and storage, advanced battery materials, and photoelectrochemistry. He has published over 290 papers in international, peer-reviewed journals with more than 18,000 citations and h-index of 72.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Sun, M., Zhang, G., Liu, H. et al. α- and γ-Fe2O3 nanoparticle/nitrogen doped carbon nanotube catalysts for high-performance oxygen reduction reaction. Sci. China Mater. 58, 683–692 (2015). https://doi.org/10.1007/s40843-015-0082-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-015-0082-x