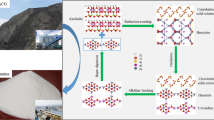
Abstract
A novel technology of hematite involved roasting-alkaline leaching-Bayer digestion process was proposed to extract alumina from high-alumina coal gangue (HACG), however the resource utilization of silica was not yet resolved. In this work, the alkaline leaching behavior of silica solid solutions in the product obtained by roasting the mixture of HACG and hematite was systematically studied in sodium hydroxide solution and sodium silicate solution, meanwhile the phase transformation was investigated by X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), scanning electronic microscope (SEM), and energy dispersive spectrometer (EDS). The results show that kaolinite, which is the main mineral in HACG, was converted into silica solid solutions (i.e., quartz solid solution and cristobalite solid solution) and alumina (i.e., θ-Al2O3 and α-Al2O3) through reductively roasting with hematite followed by oxidation during cooling process. Elevated oxidation temperature promotes the conversion of θ-Al2O3 into α-Al2O3. The silica solid solutions were readily soluble in sodium hydroxide solution and sodium silicate solution while α-Al2O3 was stable, hence, efficient separation of silica and alumina. Through leaching in sodium silicate solution with a modulus of ~ 1.0 followed by sodium hydroxide solution, a sodium silicate solution with a modulus of ~ 2.5 was obtained together with an alumina concentrate with a mass ratio of alumina to silica of > 18.0. The alumina concentrate is a decent raw material for alumina extraction by Bayer digestion, and the sodium silicate solution with a modulus of ~ 2.5 can be used in the chemical industry.
Graphical Abstract

















Similar content being viewed by others
References
United States Geological Survey (USGS) (2020) Mineral commodity summaries: bauxite and alumina. https://pubs.usgs.gov/periodicals/mcs2020/mcs2020-bauxite-alumina.pdf
Thomas LP (2013) Coal resources and reserves. In: Osborne D (ed) The coal handbook: towards cleaner production. Woodhead Publishing, Sawston, pp 80–106
Li J, Wang J (2019) Comprehensive utilization and environmental risks of coal gangue: a review. J Cleaner Prod 239:117946. https://doi.org/10.1016/j.jclepro.2019.117946
Moghadam MJ, Ajalloeian R, Hajiannia A (2019) Preparation and application of alkali-activated materials based on waste glass and coal gangue: a review. Constr Build Mater 221:84–98. https://doi.org/10.1016/j.conbuildmat.2019.06.071
Li F, Fang Y (2016) Ash fusion characteristics of a high aluminum coal and its modification. Energy Fuels 30(4):2925–2931. https://doi.org/10.1021/ACS.ENERGYFUELS.6B00285
Yao ZT, Ji XS, Sarker PK et al (2015) A comprehensive review on the applications of coal fly ash. Earth Sci Rev 141:105–121. https://doi.org/10.1016/j.earscirev.2014.11.016
Valeev D, Bobylev P, Osokin N et al (2022) A review of the alumina production from coal fly ash, with a focus in Russia. J Clean Prod 363:132360. https://doi.org/10.1016/j.jclepro.2022.132360
Xiao J, Li F, Zhong Q et al (2015) Separation of aluminum and silica from coal gangue by elevated temperature acid leaching for the preparation of alumina and SiC. Hydrometallurgy 155:118–124. https://doi.org/10.1016/j.hydromet.2015.04.018
Smith P (2009) The processing of high silica bauxites—review of existing and potential processes. Hydrometallurgy 98(1–2):162–176. https://doi.org/10.1016/j.hydromet.2009.04.015
Li XB, Wang HY et al (2019) Efficient separation of silica and alumina in simulated CFB slag by reduction roasting-alkaline leaching process. Waste Manag 87:798–804. https://doi.org/10.1016/j.wasman.2019.03.020
Wang H, Zhang X, Liu C et al (2021) Comprehensive extraction of silica and alumina from high-alumina coal gangue (HACG): hematite involved roasting—alkaline leaching—Bayer digestion process. J Sustain Metall 7(4):1686–1698. https://doi.org/10.1007/s40831-021-00433-4
Vinai R, Soutsos M (2019) Production of sodium silicate powder from waste glass cullet for alkali activation of alternative binders. Cem Concr Res 116:45–56. https://doi.org/10.1016/j.cemconres.2018.11.008
Lian X, Peng ZH, Shen LT et al (2021) Properties of low-modulus sodium silicate solution in alkali system. Trans Nonferrous Met Soc China 31(12):3918–3928. https://doi.org/10.1016/S1003-6326(21)65774-6
Li XB, Gao XB, Wang YL et al (2022) Coal fly ash cleaner utilization by ferric oxide assisted roasting—leaching silica: recycling lixiviant by seeded precipitation of leachate. Process Saf Environ Prot 164:827–835. https://doi.org/10.1016/j.psep.2022.06.068
Li XB, Wang HY, Zhou QS et al (2019) Reaction behavior of kaolinite with ferric oxide during reduction roasting. Trans Nonferrous Met Soc China 29(1):186–193. https://doi.org/10.1016/S1003-6326(18)64927-1
Watts HL, Utley DW (1953) Volumetric analysis of sodium aluminate solutions. Anal Chem 25(6):864–867. https://doi.org/10.1021/ac60078a005
Li XB, Wang HY, Zhou QS et al (2020) Phase transformation of hercynite during the oxidative roasting process. JOM 72(10):3341–3347. https://doi.org/10.1007/s11837-020-04215-3
Wang HY, Zhang XX, Yang SY et al (2022) Separation of alumina and silica from metakaolinite by reduction roasting-alkaline leaching process: effect of CaSO4 and CaO. Trans Nonferrous Met Soc China 32(3):999–1009. https://doi.org/10.1016/S1003-6326(22)65849-7
Lunevich L, Sanciolo P, Dumee LF (2016) Silica fouling in high salinity waters in reverse osmosis desalination (sodium-silica system). Environ Sci Water Res 2:539–548. https://doi.org/10.1039/C6EW00065G
Yang XH, Zhu WL, Yang Q (2008) The viscosity properties of sodium silicate solutions. J Solut Chem 37(1):73–83. https://doi.org/10.1007/s10953-007-9214-6
Nordström J, Nilsson E, Jarvol P et al (2011) Concentration- and pH-dependence of highly alkaline sodium silicate solutions. J Colloid Interface Sci 356(1):37–45. https://doi.org/10.1016/j.jcis.2010.12.085
Croker D, Loan M, Hodnett BK (2008) Desilication reactions at digestion conditions: an in situ X-ray diffraction study. Cryst Growth Des 8(12):4499–4505. https://doi.org/10.1021/cg8004739
Jastrzębska I, Szczerba J, Błachowski A et al (2017) Structure and microstructure evolution of hercynite spinel (Fe2+Al2O4) after annealing treatment. Eur J Mineral 29(1):63–72. https://doi.org/10.1127/ejm/2017/0029-2579
Nauman RV, Debye P (1951) Light-scattering investigations of carefully filtered sodium silicate solutions. J Phys Colloid Chem 55(1):1–9. https://doi.org/10.1021/j150484a001
Wang RC, Zhai YC, Ning ZQ et al (2014) Kinetics of SiO2 leaching from Al2O3 extracted slag of fly ash with sodium hydroxide solution. Trans Nonferrous Met Soc China 24(6):1928–1936. https://doi.org/10.1016/S1003-6326(14)63273-8
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 52004194) and the Postdoctoral Research Foundation of China (Grant No. 2019M662733).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
The contributing editor for this article was Atsushi Shibayama.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Xb., Wang, P., Wang, Hy. et al. The Alkaline Leaching Behavior of Silica Solid Solutions in the Product Obtained by Roasting the Mixture of High-Alumina Coal Gangue and Hematite. J. Sustain. Metall. 8, 1853–1865 (2022). https://doi.org/10.1007/s40831-022-00615-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-022-00615-8