Abstract
Background
Many patients with primary membranous nephropathy have severe proteinuria unresponsive to optimized renin–angiotensin–aldosterone system inhibitors (RAASi). We evaluated the efficacy and safety of hydroxychloroquine as an adjunctive agent in membranous nephropathy (MN) treatments.
Methods
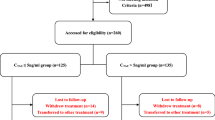
We prospectively recruited 126 patients with biopsy-proven primary membranous nephropathy and urinary protein 1–8 g/day while receiving optimized RAASi treatment for ≥ 3 months and well-controlled blood pressure. Forty-three patients received hydroxychloroquine and RAASi (hydroxychloroquine-RAASi group), and 83 patients received RAASi alone (RAASi group). Treatment responses, including proteinuria reduction, complete and partial remission rates, and autoantibody against phospholipase A2 receptor (anti-PLA2R) levels, were compared between the two groups at 6 months and over the long term.
Results
At 6 months, the effective response rate (proteinuria reduction > 30%) (57.5% vs. 28.9%, P = 0.002), clinical remission rate (35.0% vs. 15.7%, P = 0.015), and percentage change in proteinuria (− 51.7% vs. − 21.9%, P < 0.001) were higher, and the rate of switching to immunosuppressants (25.0% vs. 45.8%, P = 0.027) was lower in the hydroxychloroquine-RAASi group than in the RAASi group. Hydroxychloroquine administration was an independent protective factor with an effective response (OR 0.37, P = 0.021). In the long term, the clinical remission rate was higher in the HCQ-RAASi group (62.5% vs. 38.6%, P = 0.013). Hydroxychloroquine therapy was associated with a higher rate of anti-PLA2R reduction (< 20 U/ml) (HR 0.28, P = 0.031). We observed no serious adverse events associated with hydroxychloroquine.
Conclusions
Hydroxychloroquine could be an option for patients with membranous nephropathy seeking to achieve proteinuria reduction and anti-PLA2R antibody reduction in addition to optimized RAASi. Randomized controlled trials are needed to confirm these findings.
Trial registration
ChiCTR2100045947, 20210430, retrospectively registered.




Similar content being viewed by others
Availability of data and material
The datasets used or analyzed during the current study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
References
Fogo AB, Lusco MA, Najafian B, Alpers CE (2015) AJKD atlas of renal pathology: membranous nephropathy. Am J Kidney Dis 66(3):e15–e17. https://doi.org/10.1053/j.ajkd.2015.07.006 ((Epub 2015/08/25, PubMed PMID: 26300203))
Beck LH, Bonegio RGB, Lambeau G, Beck DM, Powell DW, Cummins TD et al (2009) M-type phospholipase A (sub 2) receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361(1):11–21. https://doi.org/10.1056/NEJMoa0810457 ((PubMed PMID: WOS:000267533100005))
Radice A, Pieruzzi F, Trezzi B, Ghiggeri G, Napodano P, D’Amico M et al (2018) Diagnostic specificity of autoantibodies to M-type phospholipase A2 receptor (PLA2R) in differentiating idiopathic membranous nephropathy (IMN) from secondary forms and other glomerular diseases. J Nephrol 31(2):271–278. https://doi.org/10.1007/s40620-017-0451-5 ((Epub 2017/10/31, PubMed PMID: 29081027))
Meyer-Schwesinger C, Lambeau G, Stahl RA (2015) Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 372(11):1074–1075. https://doi.org/10.1056/NEJMc1500130 ((Epub 2015/03/12, PubMed PMID: 25760364))
Sethi S, Madden BJ, Debiec H, Charlesworth MC, Gross L, Ravindran A et al (2019) Exostosin 1/exostosin 2-associated membranous nephropathy. J Am Soc Nephrol 30(6):1123–1136. https://doi.org/10.1681/ASN.2018080852 ((Epub 2019/05/08, PubMed PMID: 31061139; PubMed Central PMCID: PMCPMC6551791))
Sethi S, Debiec H, Madden B, Charlesworth MC, Morelle J, Gross L et al (2020) Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int 97(1):163–174. https://doi.org/10.1016/j.kint.2019.09.014 ((Epub 2020/01/07, PubMed PMID: 31901340))
Sethi S, Debiec H, Madden B, Vivarelli M, Charlesworth MC, Ravindran A et al (2020) Semaphorin 3B-associated membranous nephropathy is a distinct type of disease predominantly present in pediatric patients. Kidney Int 98(5):1253–1264. https://doi.org/10.1016/j.kint.2020.05.030 ((Epub 2020/06/14, PubMed PMID: 32534052))
Sethi S, Madden B, Debiec H, Morelle J, Charlesworth MC, Gross L et al (2021) Protocadherin 7-associated membranous nephropathy. J Am Soc Nephrol 32(5):1249–1261. https://doi.org/10.1681/asn.2020081165 ((Epub 2021/04/10, PubMed PMID: 33833079; PubMed Central PMCID: PMCPMC8259689))
Caza TN, Hassen SI, Kuperman M, Sharma SG, Dvanajscak Z, Arthur J et al (2021) Neural cell adhesion molecule 1 is a novel autoantigen in membranous lupus nephritis. Kidney Int 100(1):171–181. https://doi.org/10.1016/j.kint.2020.09.016 ((Epub 2020/10/13, PubMed PMID: 33045259; PubMed Central PMCID: PMCPMC8032825))
Jefferson JA (2018) Complications of immunosuppression in glomerular disease. Clin J Am Soc Nephrol 13(8):1264–1275. https://doi.org/10.2215/CJN.01920218 ((Epub 2018/07/26, PubMed PMID: 30042223; PubMed Central PMCID: PMCPMC6086710))
Polanco N, Gutiérrez E, Rivera F, Castellanos I, Baltar J, Lorenzo D et al (2012) Spontaneous remission of nephrotic syndrome in membranous nephropathy with chronic renal impairment. Nephrol Dial Transplant 27(1):231–234. https://doi.org/10.1093/ndt/gfr285 ((Epub 2011/06/01, PubMed PMID: 21624942))
Cattran DC, Feehally J, Cook HT, Liu ZH, Fervenza FC, Mezzano SA et al (2012) Kidney disease: improving global outcomes (KDIGO) glomerulonephritis work group. KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl 2(2):139–274. https://doi.org/10.1038/kisup.2012.9
van den Brand JA, van Dijk PR, Hofstra JM, Wetzels JF (2014) Long-term outcomes in idiopathic membranous nephropathy using a restrictive treatment strategy. J Am Soc Nephrol 25(1):150–158. https://doi.org/10.1681/ASN.2013020185 ((Epub 2013/09/14, PubMed PMID: 24029426; PubMed Central PMCID: PMCPMC3871776))
Hladunewich MA, Troyanov S, Calafati J, Cattran DC, Metropolitan Toronto Glomerulonephritis R (2009) The natural history of the non-nephrotic membranous nephropathy patient. Clin J Am Soc Nephrol 4(9):1417–1422. https://doi.org/10.2215/CJN.01330209 ((Epub 2009/08/08, PubMed PMID: 19661220; PubMed Central PMCID: PMCPMC2736692))
Vogt L, Navis G, de Zeeuw D (2005) Individual titration for maximal blockade of the renin-angiotensin system in proteinuric patients: a feasible strategy? J Am Soc Nephrol 16(Suppl 1):S53–S57. https://doi.org/10.1681/asn.2004121074 ((Epub 2005/06/07, PubMed PMID: 15938035))
Tocci G, Citoni B, Presta V, Leoncini G, Viazzi F, Bonino B et al (2020) Effects of dual inhibition of renin-angiotensin-aldosterone system on cardiovascular and renal outcomes: balancing the risks and the benefits. Intern Emerg Med 15(3):373–379. https://doi.org/10.1007/s11739-019-02257-3 ((Epub 2019/12/23, PubMed PMID: 31865522))
Schrezenmeier E, Dorner T (2020) Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol 16(3):155–166. https://doi.org/10.1038/s41584-020-0372-x ((Epub 2020/02/09, PubMed PMID: 32034323))
Ponticelli C, Moroni G (2016) Hydroxychloroquine in systemic lupus erythematosus (SLE). Expert Opin Drug Saf 16(3):411–419. https://doi.org/10.1080/14740338.2017.1269168
Wallace DJ, Gudsoorkar VS, Weisman MH, Venuturupalli SR (2012) New insights into mechanisms of therapeutic effects of antimalarial agents in SLE. Nat Rev Rheumatol 8(9):522–533. https://doi.org/10.1038/nrrheum.2012.106 ((Epub 2012/07/18, PubMed PMID: 22801982))
Kasitanon N, Fine DM, Haas M, Magder LS, Petri M (2006) Hydroxychloroquine use predicts complete renal remission within 12 months among patients treated with mycophenolate mofetil therapy for membranous lupus nephritis. Lupus 15(6):366–370. https://doi.org/10.1191/0961203306lu2313oa ((Epub 2006/07/13, PubMed PMID: 16830883))
Siso A, Ramos-Casals M, Bove A, Brito-Zeron P, Soria N, Munoz S et al (2008) Previous antimalarial therapy in patients diagnosed with lupus nephritis: influence on outcomes and survival. Lupus 17(4):281–288. https://doi.org/10.1177/0961203307086503 ((Epub 2008/04/17, PubMed PMID: 18413408))
Cunha C, Alexander S, Ashby D, Lee J, Chusney G, Cairns TD et al (2018) Hydroxycloroquine blood concentration in lupus nephritis: a determinant of disease outcome? Nephrol Dial Transplant 33(9):1604–1610. https://doi.org/10.1093/ndt/gfx318 ((Epub 2017/12/01, PubMed PMID: 29186572; PubMed Central PMCID: PMCPMC7170714))
Lee JS, Oh JS, Kim YG, Lee CK, Yoo B, Hong S (2020) Recovery of renal function in patients with lupus nephritis and reduced renal function: the beneficial effect of hydroxychloroquine. Lupus 29(1):52–57. https://doi.org/10.1177/0961203319890007 ((Epub 2019/12/04, PubMed PMID: 31793379))
Rodrigues JC, Bargman JM (2018) Antimalarial drugs for the prevention of chronic kidney disease in patients with rheumatoid arthritis the importance of controlling chronic inflammation? Clin J Am Soc Nephrol 13(5):679–680. https://doi.org/10.2215/cjn.03300318 ((PubMed PMID: WOS:000432174800003))
Wu CL, Chang CC, Kor CT, Yang TH, Chiu PF, Tarng DC et al (2018) Hydroxychloroquine use and risk of CKD in patients with rheumatoid arthritis. Clin J Am Soc Nephrol 13(5):702–709. https://doi.org/10.2215/CJN.11781017 ((Epub 2018/04/18, PubMed PMID: 29661770; PubMed Central PMCID: PMCPMC5969483))
Gao RT, Wu W, Wen YB, Li XM (2017) Hydroxychloroquine alleviates persistent proteinuria in IgA nephropathy. Int Urol Nephrol 49(7):1233–1241. https://doi.org/10.1007/s11255-017-1574-2 ((PubMed PMID: WOS:000403494000017))
Liu LJ, Yang YZ, Shi SF, Bao YF, Yang C, Zhu SN et al (2019) Effects of hydroxychloroquine on proteinuria in IgA nephropathy: a randomized controlled trial. Am J Kidney Dis 74(1):15–22. https://doi.org/10.1053/j.ajkd.2019.01.026 ((PubMed PMID: WOS:000472166000006))
Yang YZ, Chen P, Liu LJ, Cai QQ, Shi SF, Chen YQ et al (2019) Comparison of the effects of hydroxychloroquine and corticosteroid treatment on proteinuria in IgA nephropathy: a case-control study. Bmc Nephrol. https://doi.org/10.1186/s12882-019-1488-6 ((PubMed PMID: WOS:000480272600002))
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006 ((Epub 2009/05/06, PubMed PMID: 19414839; PubMed Central PMCID: PMCPMC2763564))
Tseng DS, Kwong J, Rezvani F, Coates AO (2010) Angiotensin-converting enzyme-related Cough among Chinese–Americans. Am J Med 123(2):183.e11–e15. https://doi.org/10.1016/j.amjmed.2009.06.032
Andersen S, Rossing P, Juhl TR, Deinum J, Parving HH (2002) Optimal dose of losartan for renoprotection in diabetic nephropathy. Nephrol Dial Transplant 17(8):1413–1418. https://doi.org/10.1093/ndt/17.8.1413 ((Epub 2002/07/31, PubMed PMID: 12147788))
Andersen S, Tarnow L, Rossing P, Hansen BV, Parving HH (2000) Renoprotective effects of angiotensin II receptor blockade in type 1 diabetic patients with diabetic nephropathy. Kidney Int 57(2):601–606. https://doi.org/10.1046/j.1523-1755.2000.00880.x ((Epub 2000/01/29, PubMed PMID: 10652037))
Laverman GD, Henning RH, de Jong PE, Navis G, de Zeeuw D (2001) Optimal antiproteinuric dose of losartan in nondiabetic patients with nephrotic range proteinuria. Am J Kidney Dis 38(6):1381–1384. https://doi.org/10.1053/ajkd.2001.29262 ((Epub 2001/12/01, PubMed PMID: 11728979))
Pons-Estel GJ, Alarcon GS, Burgos PI, Hachuel L, Boggio G, Wojdyla D et al (2013) Mestizos with systemic lupus erythematosus develop renal disease early while antimalarials retard its appearance: data from a Latin American cohort. Lupus 22(9):899–907. https://doi.org/10.1177/0961203313496339 ((Epub 2013/07/17, PubMed PMID: 23857989; PubMed Central PMCID: PMCPMC3943422))
Okpechi IG, Ayodele OE, Jones ES, Duffield M, Swanepoel CR (2012) Outcome of patients with membranous lupus nephritis in Cape Town South Africa. Nephrol Dial Transplant 27(9):3509–3515. https://doi.org/10.1093/ndt/gfs122 ((Epub 2012/05/23, PubMed PMID: 22610989))
Koh JH, Ko HS, Kwok SK, Ju JH, Park SH (2015) Hydroxychloroquine and pregnancy on lupus flares in Korean patients with systemic lupus erythematosus. Lupus 24(2):210–217. https://doi.org/10.1177/0961203314555352 ((Epub 2014/10/12, PubMed PMID: 25305214))
Beck LH Jr, Fervenza FC, Beck DM, Bonegio RGB, Malik FA, Erickson SB et al (2011) Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J Am Soc Nephrol 22(8):1543–1550. https://doi.org/10.1681/ASN.2010111125 ((Epub 2011/07/26, PubMed PMID: 21784898; PubMed Central PMCID: PMCPMC3148709))
Ruggenenti P, Debiec H, Ruggiero B, Chianca A, Pelle T, Gaspari F et al (2015) Anti-phospholipase A(2) receptor antibody titer predicts post-rituximab outcome of membranous nephropathy. J Am Soc Nephrol 26(10):2545–2558. https://doi.org/10.1681/asn.2014070640 ((PubMed PMID: WOS:000362156700026))
De Vriese AS, Glassock RJ, Nath KA, Sethi S, Fervenza FC (2017) A proposal for a serology-based approach to membranous nephropathy. J Am Soc Nephrol 28(2):421–430. https://doi.org/10.1681/asn.2016070776 ((PubMed PMID: WOS:000393017600007))
Mauthe M, Orhon I, Rocchi C, Zhou X, Luhr M, Hijlkema KJ et al (2018) Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 14(8):1435–1455. https://doi.org/10.1080/15548627.2018.1474314 ((Epub 2018/06/27, PubMed PMID: 29940786; PubMed Central PMCID: PMCPMC6103682))
Al-Bari MA (2015) Chloroquine analogues in drug discovery: new directions of uses, mechanisms of actions and toxic manifestations from malaria to multifarious diseases. J Antimicrob Chemother 70(6):1608–1621. https://doi.org/10.1093/jac/dkv018 ((Epub 2015/02/20, PubMed PMID: 25693996))
Van de Logt AE, Fresquet M, Wetzels JF, Brenchley P (2019) The anti-PLA2R antibody in membranous nephropathy: what we know and what remains a decade after its discovery. Kidney Int 96(6):1292–1302. https://doi.org/10.1016/j.kint.2019.07.014 ((PubMed PMID: WOS:000497968800012))
Ronco P, Debiec H (2020) Molecular pathogenesis of membranous nephropathy. Annu Rev Pathol 15:287–313. https://doi.org/10.1146/annurev-pathol-020117-043811 ((Epub 2019/10/18, PubMed PMID: 31622560))
Piconi S, Parisotto S, Rizzardini G, Passerini S, Terzi R, Argenteri B et al (2011) Hydroxychloroquine drastically reduces immune activation in HIV-infected, antiretroviral therapy-treated immunologic nonresponders. Blood 118(12):3263–3272. https://doi.org/10.1182/blood-2011-01-329060 ((Epub 2011/05/18, PubMed PMID: 21576701))
Thome R, Moraes AS, Bombeiro AL, Farias Ados S, Francelin C, da Costa TA et al (2013) Chloroquine treatment enhances regulatory T cells and reduces the severity of experimental autoimmune encephalomyelitis. PLoS ONE 8(6):e65913. https://doi.org/10.1371/journal.pone.0065913 ((Epub 2013/06/27, PubMed PMID: 23799062; PubMed Central PMCID: PMCPMC3683039))
Tselios K, Sarantopoulos A, Gkougkourelas I, Boura P (2015) The influence of therapy on CD4+CD25(high)FOXP3+ regulatory T cells in systemic lupus erythematosus patients: a prospective study. Scand J Rheumatol 44(1):29–35. https://doi.org/10.3109/03009742.2014.922214 ((Epub 2014/09/11, PubMed PMID: 25205084))
Rosenzwajg M, Languille E, Debiec H, Hygino J, Dahan K, Simon T et al (2017) B- and T-cell subpopulations in patients with severe idiopathic membranous nephropathy may predict an early response to rituximab. Kidney Int 92(1):227–237. https://doi.org/10.1016/j.kint.2017.01.012 ((Epub 2017/03/21, PubMed PMID: 28318628))
Roccatello D, Sciascia S, Di Simone D, Solfietti L, Naretto C, Fenoglio R et al (2016) New insights into immune mechanisms underlying response to Rituximab in patients with membranous nephropathy: A prospective study and a review of the literature. Autoimmun Rev 15(6):529–538. https://doi.org/10.1016/j.autrev.2016.02.014 ((PubMed PMID: WOS:000375499700005))
Acknowledgements
The technical support from Jin-ying Wang is greatly appreciated. This work was supported by grants from the Natural Science Foundation of China (81870486, 82070732, and 82090021).
Funding
Natural Science Foundation of China (81870486, 82070732, and 82090021).
Author information
Authors and Affiliations
Contributions
Research idea and study design: ZC and X-YC; data acquisition: X-YC and Y-JC; data analysis/interpretation and statistical analysis: Y-JC; supervision and mentorship: M-HZ. Each author contributed important intellectual content during the drafting and revision of the manuscript and has accepted responsibility for the overall work by ensuring that any questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. ZC takes responsibility for the following: that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Ethical approval
The research followed the Declaration of Helsinki and was approved by the ethics committee of Peking University First Hospital.
Consent to participate
Written informed consent was obtained from all individual participants.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cheng, Yj., Cheng, Xy., Zhang, Ym. et al. Effects of hydroxychloroquine on proteinuria in membranous nephropathy. J Nephrol 35, 1145–1157 (2022). https://doi.org/10.1007/s40620-021-01182-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-021-01182-z