Abstract
Background
Patients (pts) with primary Membranous nephropathy (MN) have an autoimmune disease caused by autoantibodies against podocyte antigens and 60-80% of them have antibodies directed against the M-type phospholipase A2 receptor (PLA2R). Immunosuppressive treatment is recommended in high-medium risk pts. Recently the use of rituximab (RTX), has emerged as an important therapeutic option in pts with primary MN. The appropriate cumulative dose of RTX in PMN pts is still uncertain, and favorable outcomes even with low-dose of RTX has been described. We compared efficacy and safety of 3 different treatment regimens: low-dose RTX (Protocol 1, one dose of RTX 375 mg/m2), standard RTX protocol (Protocol 2, four weekly doses of rituximab 375 mg/m2) and Ponticelli’s regimen.
Methods
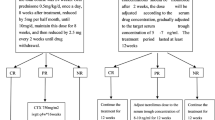
42 pts with primary MN and nephrotic syndrome were assigned to Protocol 1 (14 pts) or Protocol 2 (14 pts). All patients were followed for 24 months after RTX. Fourteen pts, matched for age and baseline serum creatinine (sCr) and proteinuria, treated with Ponticelli’s regimen were examined as controls.
Results
At 24 months, a significant improvement in proteinuria levels was observed in pts treated with Protocol 1 (7.5 ± 4.8 at T0; 0.21 ± 0.15 at T24, p < 0.01), Protocol 2 (5.1 ± 1.41 g/24 at T0; 0.35 ± 0.39 at T24 p < 0.01) and controls (8.27 ± 4.78 T0; 2.2 ± 1.9 g/24 h at T24, p < 0.01). No differences in clinical response (p = 0.53) was observed comparing the 3 groups.
Conclusions
Our data suggest that the RTX is a promising alternative to Ponticelli’s regimen even at low-doses. This makes RTX a cost-effective treatment of primary MN in the short and medium terms.




Similar content being viewed by others
References
Fervenza FC, Sethi S, Specks U (2008) Idiopathic membranous nephropathy: diagnosis and treatment. Clin J Am Soc Nephrol 3(3):905–919
Bomback AS, Fervenza FC (2018) Membranous nephropathy: approaches to treatment. Am J Nephrol 47(Suppl 1):30–42. https://doi.org/10.1159/000481635
Polanco N, Gutiérrez E, Rivera F, Castellanos I, Baltar J, Lorenzo D, Praga M, Grupo de Estudio de las Enfermedades Glomerulares de la Sociedad Española de Nefrología (GLOSEN) (2012) Spontaneous remission of nephrotic syndrome in membranous nephropathy with chronic renal impairment. Nephrol Dial Transplant 27(1):231–234. https://doi.org/10.1093/ndt/gfr285
Kanigicherla DA, Short CD, Roberts SA, Hamilton P, Nikam M, Harris S, Brenchley PE, Venning MC (2016) Long-term outcomes of persistent disease and relapse in primary membranous nephropathy. Nephrol Dial Transplant 31(12):2108–2114. https://doi.org/10.1093/ndt/gfv435
Troyanov S, Wall CA, Miller JA, Scholey JW, Cattran DC, Toronto Glomerulonephritis Registry Group (2004) Idiopathic membranous nephropathy: definition and relevance of a partial remission. Kidney Int 66(3):1199–1205. https://doi.org/10.1111/j.1523-1755.2004.00873.x
Disease K (2012) Improving global outcomes (KDIGO): KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl 2:139–274
Howman A, Chapman TL, Langdon MM, Ferguson C, Adu D, Feehally J, Gaskin GJ, Jayne DR, O'Donoghue D, Boulton-Jones M, Mathieson PW (2013) Immunosuppression for progressive membranous nephropathy: a UK randomised controlled trial. Lancet 381(9868):744–751. https://doi.org/10.1016/S0140-6736(12)61566-9
Bomback AS, Derebail VK, McGregor JG, Kshirsagar AV, Falk RJ, Nachman PH (2009) Rituximab therapy for membranous nephropathy: a systematic review. Clin J Am Soc Nephrol 4:734–744. https://doi.org/10.2215/CJN.05231008
Remuzzi G, Chiurchiu C, Abbate M et al (2002) Rituximab for idiopathic membranous nephropathy. Lancet 360(9337):923–924 (Erratum in: Lancet 2002 Dec 21-28;360(9350):2090. https//doi.org/10.1016/S0140-6736(02)11042-7)
Xu P, He YD, Yu ZM, Luo K, Xie HY, Zou PM, Gu X, Wang SR, Cai JF, Xu Q, Li H, Li XW (2018) Therapy of rituximab in idiopathic membranous nephropathy with nephrotic syndrome: a systematic review and meta-analysis. Chin Med Sci J 33(1):9–19. https://doi.org/10.24920/21803
Roccatello D, Sciascia S, Di Simone D, Solfietti L, Naretto C, Fenoglio R, Baldovino S, Menegatti E (2016) New insights into immune mechanisms underlying response to rituximab in patients with membranous nephropathy: a prospective study and a review of the literature. Autoimmun Rev 15(6):529–538. https://doi.org/10.1016/j.autrev.2016.02.014
Rosenzwajg M, Languille E, Debiec H, Hygino J, Dahan K, Simon T, Klatzmann D, Ronco P et al (2017) Kidney Int 92(1):227–237. https://doi.org/10.1016/j.kint.2017.01.01
Fervenza FC, Appel GB, Barbour SJ, MENTOR Investigators et al (2019) Rituximab or cyclosporine in the treatment of membranous nephropathy. N Engl J Med 381(1):36–46. https://doi.org/10.1056/NEJMoa1814427(Treatment of idiopathic membranous nephropathy (MENTOR). Nephron 130(3):159–168)
Rojas-Rivera J, Fernández-Juárez G, Ortiz A, Hofstra J, Gesualdo L, Tesar V, Wetzels J, Segarra A, Egido J, Praga M (2015) A European multicentre and open-label controlled randomized trial to evaluate the efficacy of sequential treatment with tacrolimus-rituximab versus steroids plus cyclophosphamide in patients with primary membranous nephropathy: the STARMEN study. Clin Kidney J 8(5):503–510. https://doi.org/10.1093/ckj/sfv075
Cravedi P, Ruggenenti P, Sghirlanzoni MC, Remuzzi G (2007) Titrating rituximab to circulating B cells to optimize lymphocytolytic therapy in idiopathic membranous nephropathy. Clin J Am Soc Nephrol 2(5):93
Ruggenenti P, Cravedi P, Remuzzi G (2009) Rituximab for membranous nephropathy and immune disease: less might be enough. Nat Clin Pract Nephrol 5(2):76–77
Bagchi S, Subbiah AK, Bhowmik D, Mahajan S, Yadav RK, Kalaivani M, Singh G, Dinda A, Kumar Agarwal S (2018) Low-dose rituximab therapy in resistant idiopathic membranous nephropathy: single-center experience. Clin Kidney J 11(3):337–341. https://doi.org/10.1093/ckj/sfx105
Moroni G, Depetri F, Del Vecchio L, Gallelli B, Raffiotta F, Giglio E, Brunini F, D'Amico M, Longhi S, Radice A, Messa P, Sinico RA (2017) Low-dose rituximab is poorly effective in patients with primary membranous nephropathy. Nephrol Dial Transplant 32(10):1691–1696. https://doi.org/10.1093/ndt/gfw251
Seitz-Polski B, Dahan K, Debiec H, Rousseau A, Andreani M, Zaghrini C, Ticchioni M, Rosenthal A, Benzaken S, Bernard G, Lambeau G, Ronco P, Esnault VLM (2009) High-dose rituximab and early remission in pla2r1-related membranous nephropathy. Clin J Am Soc Nephrol 4(8):1173–1182. https://doi.org/10.2215/CJN.11791018
Austin PC (2010) The performance of different propensity-score methods for estimating differences in proportions (risk differences or absolute risk reductions) in observational studies. Stat Med 29:2137–2148
Durrleman S, Simon R (1989) Flexible regression models with cubic splines. Stat Med 8:551–561
Austin PC (2011) Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 10:150–161
Roopenian DC, Akilesh S (2007) FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol 7:715–725. https://doi.org/10.1038/nri215529
Kuo TT, Aveson VG (2011) Neonatal Fc receptor and IgG-based therapeutics. MAbs 3:422–430. https://doi.org/10.4161/mabs.3.5.16983
Deng R, Meng YG, Hoyte K, Lutman J, Lu Y, Iyer S, DeForge LE, Theil FP, Fielder PJ, Prabhu S (2012) Subcutaneous bioavailability of therapeutic antibodies as a function of FcRn binding affinity in mice. MAbs 4:101–109. https://doi.org/10.4161/mabs.4.1.18543
Hamilton P, Kanigicherla D, Venning M, Brenchley P, Meads D (2010) Mayo Nephrology Collaborative Group. Rituximab therapy in idiopathic membranous nephropathy: a 2-year study. Clin J Am Soc Nephrol 5(12):2188–2198. https://doi.org/10.2215/CJN.05080610
Fervenza FC, Appel GB, Barbour SJ, Rovin BH, Lafayette RA, Aslam N, Jefferson JA, Gipson PE, Rizk DV, Sedor JR, Simon JF, McCarthy ET, Brenchley P, Sethi S, Avila-Casado C, Beanlands H, Lieske JC, Philibert D, Li T, Thomas LF, Green DF, Juncos LA, Beara-Lasic L, Blumenthal SS, Sussman AN, Erickson SB, Hladunewich M, Canetta PA, Hebert LA, Leung N, Radhakrishnan J, Reich HN, Parikh SV, Gipson DS, Lee DK, da Costa BR, Jüni P, Cattran DC, MENTOR Investigators (2019) Rituximab or cyclosporine in the treatment of membranous nephropathy. N Engl J Med 381(1):36–46. https://doi.org/10.1056/NEJMoa1814427
Dahan K, Debiec H, Plaisier E, Cachanado M, Rousseau A, Wakselman L, Michel PA, Mihout F, Dussol B, Matignon M, Mousson C, Simon T, Ronco P, GEMRITUX Study Group (2017) Rituximab for severe membranous nephropathy: a 6-month trial with extended follow-up. Am Soc Nephrol J 28(1):348–358. https://doi.org/10.1681/ASN.2016040449
Hamilton P, Kanigicherla D, Venning M, Brenchley P, Meads D (2018) Rituximab versus the modified Ponticelli regimen in the treatment of primary membranous nephropathy: a health economic model. Nephrol Dial Transplant 33(12):2145–2155. https://doi.org/10.1093/ndt/gfy049
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declares that they have no conflict of interest.
Ethical approval
This study was conducted retrospectively from data obtained for clinical purposes and it did not need ethical approval.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fenoglio, R., Baldovino, S., Sciascia, S. et al. Efficacy of low or standard rituximab-based protocols and comparison to Ponticelli’s regimen in membranous nephropathy. J Nephrol 34, 565–571 (2021). https://doi.org/10.1007/s40620-020-00781-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-020-00781-6