Abstract
Purpose
Non-alcoholic fatty liver disease (NAFLD) is considered as both a vital risk factor and a consequence of type 2 diabetes mellitus (T2DM). Low total testosterone (TT) is common in men with T2DM, contributing to increased risks of metabolic diseases. This study aimed to investigate the association between TT levels and the prevalence of NAFLD in men with T2DM.
Methods
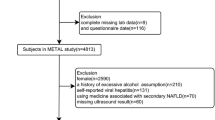
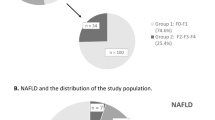
In this cross-sectional study, 1005 men with T2DM were enrolled in National Metabolic Management Center (MMC) of First Affiliated Hospital of Wenzhou Medical University between January 2017 and August 2021. NAFLD was diagnosed using ultrasound as described by the Chinese Liver Disease Association. Overweight/obesity was defined as body mass index (BMI) ≥ 25 kg/m2 according to WHO BMI classifications.
Results
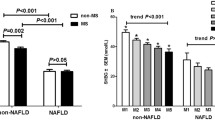
Individuals without NAFLD had higher serum TT levels than those with NAFLD. After adjustments for potential confounding factors, the top tertile was significantly associated with lower prevalence of NAFLD compared with the bottom tertile of TT level [odds ratio (OR) 0.303, 95% confidence interval (CI) 0.281–0.713; P < 0.001]. The association between TT with NAFLD in individuals with normal weight (OR 0.175, 95% CI 0.098–0.315; P < 0.001) was stronger than in individuals with overweight/obesity (OR 0.509, 95% CI 0.267–0.971; P = 0.040). There was a significant interaction of TT with overweight/obesity (P for interaction = 0.018 for NAFLD).
Conclusion
Higher serum TT was significantly associated with a lower prevalence of NAFLD in men with T2DM. We found that the relationship of TT and NAFLD was stronger in individuals with non-overweight/obesity.

Similar content being viewed by others
Availability of data and materials
All the data collected and analyzed of this study was contained in this article.
References
Zheng KI, Eslam M, George J, Zheng MH (2020) When a new definition overhauls perceptions of MAFLD related cirrhosis care. Hepatobiliary Surg Nutr 9(6):801–804. https://doi.org/10.21037/hbsn-20-725
Younossi ZM (2019) Non-alcoholic fatty liver disease—a global public health perspective. J Hepatol 70(3):531–544. https://doi.org/10.1016/j.jhep.2018.10.033
Fan JG, Kim SU, Wong VW (2017) New trends on obesity and NAFLD in Asia. J Hepatol 67(4):862–873. https://doi.org/10.1016/j.jhep.2017.06.003
Buzzetti E, Pinzani M, Tsochatzis EA (2016) The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 65(8):1038–1048. https://doi.org/10.1016/j.metabol.2015.12.012
Polyzos SA, Kountouras J, Zavos Ch (2009) The multi-hit process and the antagonistic roles of tumor necrosis factor-alpha and adiponectin in non-alcoholic fatty liver disease. Hippokratia 13(2):127
Adams LA, Waters OR, Knuiman MW, Elliott RR, Olynyk JK (2009) NAFLD as a risk factor for the development of diabetes and the metabolic syndrome: an eleven-year follow-up study. Am J Gastroenterol 104(4):861–867. https://doi.org/10.1038/ajg.2009.67
Schindhelm RK, Heine RJ, Diamant M (2007) Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care 30(9):e94. https://doi.org/10.2337/dc07-0982. (author reply e95)
Harrison SA, Gawrieh S, Roberts K, Lisanti CJ, Schwope RB, Cebe KM et al (2021) Prospective evaluation of the prevalence of non-alcoholic fatty liver disease and steatohepatitis in a large middle-aged US cohort. J Hepatol 75(2):284–291. https://doi.org/10.1016/j.jhep.2021.02.034
Ding EL, Song Y, Malik VS, Liu S (2006) Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 295(11):1288–1299. https://doi.org/10.1001/jama.295.11.1288
Zitzmann M (2009) Testosterone deficiency, insulin resistance and the metabolic syndrome. Nat Rev Endocrinol 5(12):673–681. https://doi.org/10.1038/nrendo.2009.212
Kelly DM, Nettleship JE, Akhtar S, Muraleedharan V, Sellers DJ, Brooke JC et al (2014) Testosterone suppresses the expression of regulatory enzymes of fatty acid synthesis and protects against hepatic steatosis in cholesterol-fed androgen deficient mice. Life Sci 109(2):95–103. https://doi.org/10.1016/j.lfs.2014.06.007
Barbonetti A, Caterina Vassallo MR, Cotugno M, Felzani G, Francavilla S, Francavilla F (2016) Low testosterone and non-alcoholic fatty liver disease: evidence for their independent association in men with chronic spinal cord injury. J Spinal Cord Med 39(4):443–449. https://doi.org/10.1179/2045772314Y.0000000288
Phan H, Richard A, Lazo M, Nelson WG, Denmeade SR, Groopman J et al (2021) The association of sex steroid hormone concentrations with non-alcoholic fatty liver disease and liver enzymes in US men. Liver Int 41(2):300–310. https://doi.org/10.1111/liv.14652
Sookoian S, Pirola CJ (2017) Systematic review with meta-analysis: risk factors for non-alcoholic fatty liver disease suggest a shared altered metabolic and cardiovascular profile between lean and obese patients. Aliment Pharmacol Ther 46(2):85–95. https://doi.org/10.1111/apt.14112
Tian GX, Sun Y, Pang CJ, Tan AH, Gao Y, Zhang HY et al (2012) Oestradiol is a protective factor for non-alcoholic fatty liver disease in healthy men. Obes Rev 13(4):381–387. https://doi.org/10.1111/j.1467-789X.2011.00978.x
Apostolov R, Gianatti E, Wong D, Kutaiba N, Gow P, Grossmann M et al (2022) Testosterone therapy reduces hepatic steatosis in men with type 2 diabetes and low serum testosterone concentrations. World J Hepatol 14(4):754–765. https://doi.org/10.4254/wjh.v14.i4.754
Maseroli E, Comeglio P, Corno C, Cellai I, Filippi S, Mello T et al (2021) Testosterone treatment is associated with reduced adipose tissue dysfunction and nonalcoholic fatty liver disease in obese hypogonadal men. J Endocrinol Invest 44(4):819–842. https://doi.org/10.1007/s40618-020-01381-8
Zhang Y, Wang W, Ning G (2019) Metabolic management center: an innovation project for the management of metabolic diseases and complications in China. J Diabetes 11(1):11–13. https://doi.org/10.1111/1753-0407.12847
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419. https://doi.org/10.1007/BF00280883
WHO Expert Consultation (2004) Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363(9403):157–163. https://doi.org/10.1016/S0140-6736(03)15268-3
Lu J, Bi Y, Wang T, Wang W, Mu Y, Zhao J et al (2014) The relationship between insulin-sensitive obesity and cardiovascular diseases in a Chinese population: results of the REACTION study. Int J Cardiol 172(2):388–394. https://doi.org/10.1016/j.ijcard.2014.01.073
Zeng MD, Li YM, Chen CW, Lu LG, Fan JG, Wang BY et al (2008) Chinese national consensus workshop on nonalcoholic fatty liver disease. Guidelines for the diagnosis and treatment of alcoholic liver disease. J Dig Dis 9(2):113–116. https://doi.org/10.1111/j.1751-2980.2008.00332.x
Kim S, Kwon H, Park JH, Cho B, Kim D, Oh SW et al (2012) A low level of serum total testosterone is independently associated with nonalcoholic fatty liver disease. BMC Gastroenterol 12:69. https://doi.org/10.1186/1471-230X-12-69
Jaruvongvanich V, Sanguankeo A, Riangwiwat T, Upala S (2017) Testosterone, sex hormone-binding globulin and nonalcoholic fatty liver disease: a systematic review and meta-analysis. Ann Hepatol 16(3):382–394. https://doi.org/10.5604/01.3001.0009.8593
Lazo M, Zeb I, Nasir K, Tracy RP, Budoff MJ, Ouyang P et al (2015) Association between endogenous sex hormones and liver fat in a multiethnic study of atherosclerosis. Clin Gastroenterol Hepatol 13(9):1686–93.e2. https://doi.org/10.1016/j.cgh.2014.12.033
Seo NK, Koo HS, Haam JH, Kim HY, Kim MJ, Park KC et al (2015) Prediction of prevalent but not incident non-alcoholic fatty liver disease by levels of serum testosterone. J Gastroenterol Hepatol 30(7):1211–1216. https://doi.org/10.1111/jgh.12935
Li Y, Liu L, Wang B, Chen D, Wang J (2015) Nonalcoholic fatty liver disease and alteration in semen quality and reproductive hormones. Eur J Gastroenterol Hepatol 27(9):1069–1073. https://doi.org/10.1097/MEG.0000000000000408
Saez-Lopez C, Villena JA, Simó R, Selva DM (2020) Sex hormone-binding globulin overexpression protects against high-fat diet-induced obesity in transgenic male mice. J Nutr Biochem 85:108480. https://doi.org/10.1016/j.jnutbio.2020.108480
Le TN, Nestler JE, Strauss JF 3rd, Wickham EP 3rd (2012) Sex hormone-binding globulin and type 2 diabetes mellitus. Trends Endocrinol Metab 23(1):32–40. https://doi.org/10.1016/j.tem.2011.09.005
Xita N, Georgiou I, Lazaros L, Psofaki V, Kolios G, Tsatsoulis A (2008) The role of sex hormone-binding globulin and androgen receptor gene variants in the development of polycystic ovary syndrome. Hum Reprod 23(3):693–698. https://doi.org/10.1093/humrep/dem382
Li C, Ford ES, Li B, Giles WH, Liu S (2010) Association of testosterone and sex hormone-binding globulin with metabolic syndrome and insulin resistance in men. Diabetes Care 33(7):1618–1624. https://doi.org/10.2337/dc09-1788
Yeap BB, Marriott RJ, Antonio L, Raj S, Dwivedi G, Reid CM et al (2022) Associations of serum testosterone and sex hormone-binding globulin with incident cardiovascular events in middle-aged to older men. Ann Intern Med 175(2):159–170. https://doi.org/10.7326/M21-0551
Di Stasi V, Maseroli E, Rastrelli G, Scavello I, Cipriani S, Todisco T et al (2021) SHBG as a marker of NAFLD and metabolic impairments in women referred for oligomenorrhea and/or hirsutism and in women with sexual dysfunction. Front Endocrinol (Lausanne). 12:641446. https://doi.org/10.3389/fendo.2021.641446
Kupelian V, Page ST, Araujo AB, Travison TG, Bremner WJ, McKinlay JB (2006) Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese men. J Clin Endocrinol Metab 91(3):843–850. https://doi.org/10.1210/jc.2005-1326
Ghanim H, Dhindsa S, Abuaysheh S, Batra M, Kuhadiya ND, Makdissi A et al (2018) Diminished androgen and estrogen receptors and aromatase levels in hypogonadal diabetic men: reversal with testosterone. Eur J Endocrinol 178(3):277–283. https://doi.org/10.1530/EJE-17-0673
Marino L, Jornayvaz FR (2015) Endocrine causes of nonalcoholic fatty liver disease. World J Gastroenterol 21(39):11053–11076. https://doi.org/10.3748/wjg.v21.i39.11053
Jones ME, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J et al (2000) Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci U S A 97(23):12735–12740. https://doi.org/10.1073/pnas.97.23.12735
Maffei L, Murata Y, Rochira V, Tubert G, Aranda C, Vazquez M et al (2004) Dysmetabolic syndrome in a man with a novel mutation of the aromatase gene: effects of testosterone, alendronate, and estradiol treatment. J Clin Endocrinol Metab 89(1):61–70. https://doi.org/10.1210/jc.2003-030313
Mårin P, Holmäng S, Jönsson L, Sjöström L, Kvist H, Holm G et al (1992) The effects of testosterone treatment on body composition and metabolism in middle-aged obese men. Int J Obes Relat Metab Disord 16(12):991–997
Selvin E, Feinleib M, Zhang L, Rohrmann S, Rifai N, Nelson WG et al (2007) Androgens and diabetes in men: results from the third national health and nutrition examination survey (NHANES III). Diabetes Care 30(2):234–238. https://doi.org/10.2337/dc06-1579
Zhang J, Zhao Y, Xu C, Hong Y, Lu H, Wu J et al (2014) Association between serum free fatty acid levels and nonalcoholic fatty liver disease: a cross-sectional study. Sci Rep 4:5832. https://doi.org/10.1038/srep05832
Eguchi Y, Mizuta T, Sumida Y, Ishibashi E, Kitajima Y, Isoda H et al (2011) The pathological role of visceral fat accumulation in steatosis, inflammation, and progression of nonalcoholic fatty liver disease. J Gastroenterol 46(Suppl 1):70–78. https://doi.org/10.1007/s00535-010-0340-3
Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC et al (2000) Role of brain insulin receptor in control of body weight and reproduction. Science 289(5487):2122–2125. https://doi.org/10.1126/science.289.5487.2122. (PMID: 11000114)
Russell SH, Small CJ, Stanley SA, Franks S, Ghatei MA, Bloom SR (2001) The in vitro role of tumour necrosis factor-alpha and interleukin-6 in the hypothalamic-pituitary gonadal axis. J Neuroendocrinol 13(3):296–301. https://doi.org/10.1046/j.1365-2826.2001.00632.x
Vignozzi L, Filippi S, Comeglio P, Cellai I, Sarchielli E, Morelli A et al (2014) Nonalcoholic steatohepatitis as a novel player in metabolic syndrome-induced erectile dysfunction: an experimental study in the rabbit. Mol Cell Endocrinol 384(1–2):143–154. https://doi.org/10.1016/j.mce.2014.01.014
Isidori AM, Caprio M, Strollo F, Moretti C, Frajese G, Isidori A et al (1999) Leptin and androgens in male obesity: evidence for leptin contribution to reduced androgen levels. J Clin Endocrinol Metab 84(10):3673–3680. https://doi.org/10.1210/jcem.84.10.6082
Acknowledgements
We sincerely thank the individuals involved in the present study. We also thank the support of all the colleagues in the Department of Endocrine and Metabolic Diseases at the First Affiliated Hospital of Wenzhou Medical University.
Funding
This work was funded by the Natural Science Foundation of Zhejiang Province (Project No. LY20H070003) and the National Natural Science Foundation of China (81900737).
Author information
Authors and Affiliations
Contributions
All authors made a significant contribution to the work reported. XJG designed the experiments. LJY, JZZ, YFZ, ZYH, LJD, XG, XYH, JL, YQL, LYP, and XXZ performed the experiments. JZZ, LJY, and XJG analyzed and interpreted the data. LJY, JZZ, and XH wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This study was approved by the Institutional Review Board of First Affiliated Hospital of Wenzhou Medical University (Project No. KY2021-173).
Informed consent
Written consent was obtained from all participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, L.J., Zhou, J.Z., Zheng, Y.F. et al. Association of non-alcoholic fatty liver disease with total testosterone in non-overweight/obese men with type 2 diabetes mellitus. J Endocrinol Invest 46, 1565–1572 (2023). https://doi.org/10.1007/s40618-023-02006-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-023-02006-6