Abstract
Purpose of the Review
In this review, the role of inflammation on tumorigenesis with the scope of its tumor-promoting role as an enabling characteristic of cancer will be discussed along with promising therapeutical strategies that target inflammatory microenvironment of tumors.
Recent Findings
The hallmarks of cancer conceptualized by Hanahan and Weinberg in 2000 structured our understanding of the common features of cancer better, yet new emerging hallmarks and enabling characteristics are being considered within this concept in recent years. Tumor-promoting inflammation is one of these characteristics that opened a new era in cancer therapy with promising results. Recent studies revealed that targeting inflammation directly or as an adjuvant therapy is a clinically significant approach to increase the efficiency of cancer treatments.
Summary
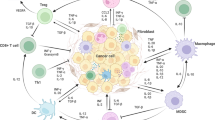
The presence of inflammatory cells in tumor development and the influence of inflammation on several cellular mechanism such as cell proliferation, invasion, and metastasis make this feature an important mediator between several hallmarks of cancer as well as a promising therapeutical target.

Similar content being viewed by others
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021. https://doi.org/10.1002/cncr.33587.
Tran KB, Lang JJ, Compton K, Xu R, Acheson AR, Henrikson HJ, et al. The global burden of cancer attributable to risk factors, 2010–19: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2022. https://doi.org/10.1016/S0140-6736(22)01438-6.
Ni J, Miller M, Stojanovic A, Garbi N, Cerwenka A. Sustained effector function of IL-12/15/18–preactivated NK cells against established tumors. J Exp Med. 2012. https://doi.org/10.1084/jem.20120944.
• Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022. https://doi.org/10.1158/2159-8290.CD-21-1059. In this review, new emerging hallmarks and enabling characteristics of cancer were proposed and the most recent information about the “hallmarks of cancer” phenomenon are discussed. This paper was integral to the current study.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011. https://doi.org/10.1016/j.cell.2011.02.013.
Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther. 2021. https://doi.org/10.1038/s41392-021-00658-5.
Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001. https://doi.org/10.1016/S0140-6736(00)04046-0.
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008. https://doi.org/10.1038/nature07205.
Hibino S, Kawazoe T, Kasahara H, Itoh S, Ishimoto T, Sakata-Yanagimoto M, et al. Inflammation-induced tumorigenesis and metastasis. Int J Mol Sci. 2021. https://doi.org/10.3390/ijms22115421.
Shchors K, Shchors E, Rostker F, Lawlor ER, Brown-Swigart L, Evan GI. The Myc-dependent angiogenic switch in tumors is mediated by interleukin 1β. Genes Dev. 2006. https://doi.org/10.1101/gad.1455706.
Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004. https://doi.org/10.1016/j.ccr.2004.09.028.
Borrello MG, Alberti L, Fischer A, Degl’Innocenti D, Ferrario C, Gariboldi M, et al. Induction of a proinflammatory program in normal human thyrocytes by the RET/PTC1 oncogene. Proc Natl Acad Sci USA. 2005. https://doi.org/10.1073/pnas.0503039102.
Guerra C, Schuhmacher AJ, Cañamero M, Grippo PJ, Verdaguer L, Pérez-Gallego L, et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007. https://doi.org/10.1016/j.ccr.2007.01.012.
Camus M, Tosolini M, Mlecnik B, Pages F, Kirilovsky A, Berger A, et al. Coordination of intratumoral immune reaction and human colorectal cancer recurrence. Cancer Res. 2009. https://doi.org/10.1158/0008-5472.CAN-08-2654.
Schumacher K, Haensch W, Röefzaad C, Schlag PM. Prognostic significance of activated CD8+ T cell infiltrations within esophageal carcinomas. Cancer Res. 2001;61(10):3932–6.
Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003. https://doi.org/10.1056/NEJMoa020177.
Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005. https://doi.org/10.1056/NEJMoa051424.
•• Friebel E, Kapolou K, Unger S, Núñez NG, Utz S, Rushing EJ, et al. Single-cell mapping of human brain cancer reveals tumor-specific instruction of tissue-invading leukocytes. Cell. 2020. https://doi.org/10.1016/j.cell.2020.04.055. This study which presents the leukocyte landscape of brain tumors was assessed by using a single-cell profiling (CyTOF) and showed a clear distinction between gliomas and brain metastases, where tissue-invading leukocytes accumulated in brain metastases regions.
Cassetta L, Pollard JW. Tumor-associated macrophages. Curr Biol. 2020. https://doi.org/10.1016/j.cub.2020.01.031.
Pan Y, Yu Y, Wang X, Zhang T. Tumor-associated macrophages in tumor immunity. Front Immunol. 2020. https://doi.org/10.3389/fimmu.2020.583084.
Hung C-N, Chen M, DeArmond DT, Chiu CH-L, Limboy CA, Tan X, et al. AXL-initiated paracrine activation of pSTAT3 enhances mesenchymal and vasculogenic supportive features of tumor-associated macrophages. Cell Rep. 2023;42:113067. http://www.ncbi.nlm.nih.gov/pubmed/37659081.
Deng Z, Shi F, Zhou Z, Sun F, Sun MH, Sun Q, et al. M1 macrophage mediated increased reactive oxygen species (ROS) influence wound healing via the MAPK signaling in vitro and in vivo. Toxicol Appl Pharmacol. 2019. https://doi.org/10.1016/j.taap.2019.01.022.
Perry CJ, Muñoz-Rojas AR, Meeth KM, Kellman LN, Amezquita RA, Thakral D, et al. Myeloid-targeted immunotherapies act in synergy to induce inflammation and antitumor immunity. J Exp Med. 2018. https://doi.org/10.1084/jem.20171435.
Hao S, Meng J, Zhang Y, Liu J, Nie X, Wu F, et al. Macrophage phenotypic mechanomodulation of enhancing bone regeneration by superparamagnetic scaffold upon magnetization. Biomaterials. 2017. https://doi.org/10.1016/j.biomaterials.2017.06.013.
Yin M, Li X, Tan S, Zhou HJ, Ji W, Bellone S, et al. Tumor-associated macrophages drive spheroid formation during early transcoelomic metastasis of ovarian cancer. J Clin Investig. 2016. https://doi.org/10.1172/JCI87252.
Sarode P, Schaefer MB, Grimminger F, Seeger W, Savai R. Macrophage and tumor cell cross-talk is fundamental for lung tumor progression: we need to talk. Front Oncol. 2020. https://doi.org/10.3389/fonc.2020.00324.
Su S, Liu Q, Chen J, Chen J, Chen F, He C, et al. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell. 2014. https://doi.org/10.1016/j.ccr.2014.03.021.
Verneau J, Sautés-Fridman C, Sun CM. Dendritic cells in the tumor microenvironment: prognostic and theranostic impact. Semin Immunol. 2020. https://doi.org/10.1016/j.smim.2020.101410.
Keirsse J, Van Damme H, Van Ginderachter JA, Laoui D. Exploiting tumor-associated dendritic cell heterogeneity for novel cancer therapies. J Leukoc Biol. 2017. https://doi.org/10.1189/jlb.4MR1116-466R.
Hsu Y-L, Huang M-S, Cheng D-E, Hung J-Y, Yang C-J, Chou S-H, et al. Lung tumor-associated dendritic cell-derived amphiregulin increased cancer progression. J Immunol. 2011. https://doi.org/10.4049/jimmunol.1100996.
Kuo CH, Chen KF, Chou SH, Huang YF, Wu CY, Cheng DE, et al. Lung tumor-associated dendritic cell-derived resistin promoted cancer progression by increasing wolf-hirschhorn syndrome candidate 1/twist pathway. Carcinogenesis. 2013. https://doi.org/10.1093/carcin/bgt281.
Kan JY, Wu DC, Yu FJ, Wu CY, Ho YW, Chiu YJ, et al. Chemokine (C-C Motif) ligand 5 is involved in tumor-associated dendritic cell-mediated colon cancer progression through non-coding RNA MALAT-1. J Cell Physiol. 2015. https://doi.org/10.1002/jcp.24918.
Kuo PL, Huang MS, Cheng DE, Hung JY, Yang CJ, Chou SH. Lung cancer-derived galectin-1 enhances tumorigenic potentiation of tumor-associated dendritic cells by expressing heparin-binding EGF-like growth factor. J Biol Chem. 2012. https://doi.org/10.1074/jbc.M111.321190.
Zitti B, Bryceson YT. Natural killer cells in inflammation and autoimmunity. Cytokine Growth Factor Rev. 2018. https://doi.org/10.1016/j.cytogfr.2018.08.001.
Bi Q, Wu JY, Qiu XM, Zhang JD, Sun ZJ, Wang W. Tumor-associated inflammation: the tumor-promoting immunity in the early stages of tumorigenesis. J Immunol Res. 2022. https://doi.org/10.1155/2022/3128933.
Speiser DE, Chijioke O, Schaeuble K, Münz C. CD4+ T cells in cancer. Nat Cancer. 2023. https://doi.org/10.1038/s43018-023-00521-2.
Hu W, Wang ZM, Feng Y, Schizas M, Hoyos BE, van der Veeken J, et al. Regulatory T cells function in established systemic inflammation and reverse fatal autoimmunity. Nat Immunol. 2021. https://doi.org/10.1038/s41590-021-01001-4.
Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression — implications for anticancer therapy. Nat Rev Clin Oncol. 2019. https://doi.org/10.1038/s41571.
Ostroumov D, Fekete-Drimusz N, Saborowski M, Kühnel F, Woller N. CD4 and CD8 T lymphocyte interplay in controlling tumor growth. Cell Mol Life Sci. 2018. https://doi.org/10.1007/s00018-017-2686-7.
Budhu A, Forgues M, Ye QH, Jia HL, He P, Zanetti KA, et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006. https://doi.org/10.1016/j.ccr.2006.06.016.
Maby P, Tougeron D, Hamieh M, Mlecnik B, Kora H, Bindea G, et al. Correlation between density of CD8+ T-cell infiltrate in microsatellite unstable colorectal cancers and frameshift mutations: a rationale for personalized immunotherapy. Cancer Res. 2015. https://doi.org/10.1158/0008-5472.CAN-14-3051.
Roberts SJ, Ng BY, Filler RB, Lewis J, Glusac EJ, Hayday AC, et al. Characterizing tumor-promoting T cells in chemically induced cutaneous carcinogenesis. Proc Natl Acad Sci USA. 2007. https://doi.org/10.1073/pnas.0604982104.
Ogura K, Sato-Matsushita M, Yamamoto S, Hori T, Sasahara M, Iwakura Y, et al. NK cells control tumor-promoting function of neutrophils in mice. Cancer Immunol Res. 2018. https://doi.org/10.1158/2326-6066.CIR-17-0204.
Køstner AH, Nielsen PS, Georgsen JB, Parner ET, Nielsen MB, Kersten C, et al. Systemic inflammation associates with a myeloid inflamed tumor microenvironment in primary resected colon cancer—may cold tumors simply be too hot? Front Immunol. 2021. https://doi.org/10.3389/fimmu.2021.716342.
Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019. https://doi.org/10.1038/s41573-018-0007-y.
Ni JJ, Zhang ZZ, Ge MJ, Chen JY, Zhuo W. Immune-based combination therapy to convert immunologically cold tumors into hot tumors: an update and new insights. Acta Pharmacol Sin. 2023. https://doi.org/10.1038/s41401-022-00953-z.
Marchesi F, Monti P, Leone BE, Zerbi A, Vecchi A, Piemonti L, et al. Increased survival, proliferation, and migration in metastatic human pancreatic tumor cells expressing functional CXCR4. Cancer Res. 2004. https://doi.org/10.1158/0008-5472.CAN-04-1343.
Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006. https://doi.org/10.1182/blood-2005-08-3182.
Koong AC, Denko NC, Hudson KM, Schindler C, Swiersz L, Koch C, et al. Candidate genes for the hypoxic tumor phenotype. Cancer Res. 2000;60(4):883–7.
Sun X, Qu Q, Lao Y, Zhang M, Yin X, Zhu H, et al. Tumor suppressor HIC1 is synergistically compromised by cancer-associated fibroblasts and tumor cells through the IL-6/pSTAT3 axis in breast cancer. BMC Cancer. 2019. https://doi.org/10.1186/s12885-019-6333-6.
Heichler C, Scheibe K, Schmied A, Geppert CI, Schmid B, Wirtz S, et al. STAT3 activation through IL-6/IL-11 in cancer-associated fibroblasts promotes colorectal tumour development and correlates with poor prognosis. Gut. 2020. https://doi.org/10.1136/gutjnl-2019-319200.
Lan T, Chen L, Wei X. Inflammatory cytokines in cancer: Comprehensive understanding and clinical progress in gene therapy. Cells. 2021. https://doi.org/10.3390/cells10010100.
Johnson DE, O’Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018. https://doi.org/10.1038/nrclinonc.2018.8.
Zhang T, Ma C, Zhang Z, Zhang H, Hu H. NF-κB signaling in inflammation and cancer. MedComm (Beijing). 2021. https://doi.org/10.1002/mco2.104.
Zhang T, Yang J, Sun Y, Song J, Gao D, Huang S, et al. Interleukin-6 and hypoxia synergistically promote EMT-mediated invasion in epithelial ovarian cancer via the IL-6/STAT3/HIF-1 α feedback loop. Anal Cell Pathol. 2023. https://doi.org/10.1155/2023/8334881.
Cornwell AC, Tisdale AA, Venkat S, Maraszek KE, Alahmari AA, George A, et al. Lorazepam stimulates IL6 production and is associated with poor survival outcomes in pancreatic cancer. Clin Cancer Res. 2023;OF1–20. https://aacrjournals.org/clincancerres/article/doi/10.1158/1078-0432.CCR-23-0547/728435/Lorazepam-Stimulates-IL6-Production-and-Is.
Laha D, Grant R, Mishra P, Nilubol N. The role of tumor necrosis factor in manipulating the immunological response of tumor microenvironment. Front Immunol. 2021. https://doi.org/10.3389/fimmu.2021.656908.
Chan FKM, Shisler J, Bixby JG, Felices M, Zheng L, Appel M, et al. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J Biol Chem. 2003. https://doi.org/10.1074/jbc.M305633200.
Niture S, Dong X, Arthur E, Chimeh U, Niture SS, Zheng W, et al. Oncogenic role of tumor necrosis factor α-induced protein 8 (TNFAIP8). Cells. 2019. https://doi.org/10.3390/cells8010009.
Poole EM, Lee IM, Ridker PM, Buring JE, Hankinson SE, Tworoger SS. A prospective study of circulating C-reactive protein, interleukin-6, and tumor necrosis factor α receptor 2 levels and risk of ovarian cancer. Am J Epidemiol. 2013. https://doi.org/10.1093/aje/kwt098.
Dobrzycka B, Terlikowski SJ, Garbowicz M, Nikliñska W, Bernaczyk PS, Nikliñski J, et al. Tumor necrosis factor-α and its receptors in epithelial ovarian cancer. Folia Histochem Cytobiol. 2009. https://doi.org/10.2478/v10042-008-0117-1.
Atretkhany KSN, Drutskaya MS, Nedospasov SA, Grivennikov SI, Kuprash DV. Chemokines, cytokines and exosomes help tumors to shape inflammatory microenvironment. Pharmacol Ther. 2016. https://doi.org/10.1016/j.pharmthera.2016.09.011.
Morgan A, Griffin M, Kameni L, Wan DC, Longaker MT, Norton JA. Medical biology of cancer-associated fibroblasts in pancreatic cancer. Biology (Basel). 2023. https://doi.org/10.3390/biology12081044.
Zhao Z, Li T, Sun L, Yuan Y, Zhu Y. Potential mechanisms of cancer-associated fibroblasts in therapeutic resistance. Biomed Pharmacothe. 2023;166:115425. https://linkinghub.elsevier.com/retrieve/pii/S0753332223012234.
Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM, et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer. 2020. https://doi.org/10.1038/s41568-019-0238-1.
You D, Wang Y, Xu J, Yang R, Wang W, Wang X, et al. MiR-3529–3p from PDGF-BB-induced cancer-associated fibroblast-derived exosomes promotes the malignancy of oral squamous cell carcinoma. Discov Oncol. 2023;14:166. https://link.springer.com/10.1007/s12672-023-00753-9.
Foster DS, Januszyk M, Delitto D, Yost KE, Griffin M, Guo J, et al. Multiomic analysis reveals conservation of cancer-associated fibroblast phenotypes across species and tissue of origin. Cancer Cell. 2022;40:1392–406.e7. https://linkinghub.elsevier.com/retrieve/pii/S1535610822004445.
Kawasaki K, Noma K, Kato T, Ohara T, Tanabe S, Takeda Y, et al. PD-L1-expressing cancer-associated fibroblasts induce tumor immunosuppression and contribute to poor clinical outcome in esophageal cancer. Cancer Immunol Immunother. 2023. https://link.springer.com/10.1007/s00262-023-03531-2.
Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010. https://doi.org/10.1016/j.cell.2010.01.025.
Brown JR, DuBois RN. COX-2: A molecular target for colorectal cancer prevention. J Clin Oncol. 2005. https://doi.org/10.1200/JCO.2005.09.051.
Stewart OA, Wu F, Chen Y. The role of gastric microbiota in gastric cancer. Gut Microbes. 2020. https://doi.org/10.1080/19490976.2020.1762520.
Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019. https://doi.org/10.5114/pg.2018.80001.
Floch P, Mégraud F, Lehours P. Helicobacter pylori strains and gastric MALT lymphoma. Toxins (Basel). 2017. https://doi.org/10.3390/toxins9040132.
Demir AB, Benvenuto D, Karacicek B, Erac Y, Spoto S, Angeletti S, et al. Implications of possible HBV-driven regulation of gene expression in stem cell-like subpopulation of Huh-7 hepatocellular carcinoma cell line. J Pers Med. 2022. https://doi.org/10.3390/jpm12122065.
Gailhouste L, Sudoh M, Qin XY, Watashi K, Wakita T, Ochiya T, et al. Epigenetic reprogramming promotes the antiviral action of IFNα in HBV-infected cells. Cell Death Discov. 2021. https://doi.org/10.1038/s41420-021-00515-y.
Hatano Y, Ideta T, Hirata A, Hatano K, Tomita H, Okada H, et al. Virus-driven carcinogenesis. Cancers (Basel). 2021. https://doi.org/10.3390/cancers13112625.
De Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005. https://doi.org/10.1016/j.ccr.2005.04.014.
Nanki K, Fujii M, Shimokawa M, Matano M, Nishikori S, Date S, et al. Somatic inflammatory gene mutations in human ulcerative colitis epithelium. Nature. 2020. https://doi.org/10.1038/s41586-019-1844-5.
Fulop T, Larbi A, Pawelec G, Khalil A, Cohen AA, Hirokawa K, et al. Immunology of aging: the birth of inflammaging. Clin Rev Allergy Immunol. 2023. https://doi.org/10.1007/s12016-021-08899-6.
Zuo L, Prather ER, Stetskiv M, Garrison DE, Meade JR, Peace TI, et al. Inflammaging and oxidative stress in human diseases: from molecular mechanisms to novel treatments. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20184472.
Di Giosia P, Stamerra CA, Giorgini P, Jamialahamdi T, Butler AE, Sahebkar A. The role of nutrition in inflammaging. Ageing Res Rev. 2022. https://doi.org/10.1016/j.arr.2022.101596.
Yang ZH, Dang YQ, Ji G. Role of epigenetics in transformation of inflammation into colorectal cancer. World J Gastroenterol. 2019. https://doi.org/10.3748/wjg.v25.i23.2863.
Chatterjee D, Das P, Chakrabarti O. Mitochondrial epigenetics regulating inflammation in cancer and aging. Front Cell Dev Biol. 2022. https://doi.org/10.3389/fcell.2022.929708.
Chumanevich AA, Spicer M, Hofseth LJ, Hébert JR. Diet, inflammation, and cancer. Diet Inflamm Health. 2022. https://doi.org/10.1158/1055-9965.EPI-19-0250.
Al Bander Z, Nitert MD, Mousa A, Naderpoor N. The gut microbiota and inflammation: an overview. Int J Environ Res Public Health. 2020. https://doi.org/10.3390/ijerph17207618.
Telle-Hansen VH, Holven KB, Ulven SM. Impact of a healthy dietary pattern on gut microbiota and systemic inflammation in humans. Nutrients. 2018. https://doi.org/10.3390/nu10111783.
Sun J, Kato I. Gut microbiota, inflammation and colorectal cancer. Genes Dis. 2016. https://doi.org/10.1016/j.gendis.2016.03.004.
Wellenstein MD, Coffelt SB, Duits DEM, van Miltenburg MH, Slagter M, de Rink I, et al. Loss of p53 triggers WNT-dependent systemic inflammation to drive breast cancer metastasis. Nature. 2019. https://doi.org/10.1038/s41586-019-1450-6.
Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019. https://doi.org/10.1016/j.immuni.2019.06.025.
Liu Y, Cao X. The origin and function of tumor-associated macrophages. Cell Mol Immunol. 2015. https://doi.org/10.1038/cmi.2014.83.
Gill JG, Piskounova E, Morrison SJ. Cancer, oxidative stress, and metastasis. Cold Spring Harb Symp Quant Biol. 2016. https://doi.org/10.1101/sqb.2016.81.030791.
Warsch W, Grundschober E, Berger A, Gille L, Cerny-Reiterer S, Tigan AS, et al. STAT5 triggers BCR-ABL1 mutation by mediating ROS production in chronic myeloid leukaemia. Oncotarget. 2012. https://doi.org/10.18632/oncotarget.806.
Shang S, Ji X, Zhang L, Chen J, Li C, Shi R, et al. Macrophage ABHD5 suppresses NFκB-dependent matrix metalloproteinase expression and cancer metastasis. Cancer Res. 2019. https://doi.org/10.1158/0008-5472.CAN-19-1059.
Tchoghandjian A, Jennewein C, Eckhardt I, Rajalingam K, Fulda S. Identification of non-canonical NF-kB signaling as a critical mediator of smac mimetic-stimulated migration and invasion of glioblastoma cells. Cell Death Dis. 2013. https://doi.org/10.1038/cddis.2013.70.
Chung SS, Aroh C, Vadgama JV. Constitutive activation of STAT3 signaling regulates hTERT and promotes stem cell-like traits in human breast cancer cells. PLoS One. 2013. https://doi.org/10.1371/journal.pone.0083971.
•• Xu Y, Ren W, Li Q, Duan C, Lin X, Bi Z, et al. LncRNA Uc003xsl.1-mediated activation of the NFkB/IL8 axis promotes progression of triple-negative breast cancer. Cancer Res. 2022; https://doi.org/10.1158/0008-5472.CAN-21-1446. In this study, a long noncoding RNA was targeted to suppress NK-kB/IL8 in triple negative breast cancer and showed that it can be a promising therapy for the treatment of this cancer type. This paper was integral to the current study.
Richmond CA, Rickner H, Shah MS, Ediger T, Deary L, Zhou F, et al. JAK/STAT-1 signaling is required for reserve intestinal stem cell activation during intestinal regeneration following acute inflammation. Stem Cell Rep. 2018. https://doi.org/10.1016/j.stemcr.2017.11.015.
Zinatizadeh MR, Schock B, Chalbatani GM, Zarandi PK, Jalali SA, Miri SR. The nuclear factor kappa B (NF-kB) signaling in cancer development and immune diseases. Genes Dis. 2021. https://doi.org/10.1016/j.gendis.2020.06.005.
Johnson DE, O’Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018. https://doi.org/10.1038/nrclinonc.2018.8.
Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T, et al. gp130-Mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009. https://doi.org/10.1016/j.ccr.2009.01.002.
Nie Y, Huang H, Guo M, Chen J, Wu W, Li W, et al. Breast phyllodes tumors recruit and repolarize tumor-associated macrophages via secreting CCL5 to promote malignant progression, which Can Be inhibited by CCR5 inhibition therapy. Clin Cancer Res. 2019. https://doi.org/10.1158/1078-0432.CCR-18-3421.
Zhu C, Mustafa D, Zheng PP, Van Der Weiden M, Sacchetti A, Brandt M, et al. Activation of CECR1 in M2-like TAMs promotes paracrine stimulation-mediated glial tumor progression. Neuro Oncol. 2017. https://doi.org/10.1093/neuonc/now251.
Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Medicine: prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-β-catenin signaling axis. Science. 2005. https://doi.org/10.1126/science.1116221.
Dufour M, Faes S, Dormond-Meuwly A, Demartines N, Dormond O. PGE2-induced colon cancer growth is mediated by mTORC1. Biochem Biophys Res Commun. 2014. https://doi.org/10.1016/j.bbrc.2014.08.032.
•• Crittenden S, Goepp M, Pollock J, Robb CT, Smyth DJ, Zhou Y, et al. Prostaglandin E2 promotes intestinal inflammation via inhibiting microbiota-dependent regulatory T cells. Sci Adv. 2021; https://doi.org/10.1126/sciadv.abd7954. In this study, it was shown that protaglandin E2, which is a mediator of inflammation, disrupt the communication between regulatory T cells and microbiota, inducing intestinal inflammation, which can trigger tumor formation. This paper was integral to the current study.
Ding Y, Zhuang S, Li Y, Yu X, Lu M, Ding N. Hypoxia-induced HIF1α dependent COX2 promotes ovarian cancer progress. J Bioenerg Biomembr. 2021. https://doi.org/10.1007/s10863-021-09900-9.
Cremoux PDE, Hamy AS, Lehmann-Che J, Scott V, Sigal B, Mathieu MC, et al. COX2/PTGS2 expression is predictive of response to neoadjuvant celecoxib in HER2-negative breast cancer patients. Anticancer Res. 2018. https://doi.org/10.21873/anticanres.12375.
Lin J, Hsiao PW, Chiu TH, Chao JI. Combination of cyclooxygenase-2 inhibitors and oxaliplatin increases the growth inhibition and death in human colon cancer cells. Biochem Pharmacol. 2005. https://doi.org/10.1016/j.bcp.2005.05.028.
Sun Y, Dai H, Chen S, Zhang Y, Wu T, Cao X, et al. Disruption of chromosomal architecture of cox2 locus sensitizes lung cancer cells to radiotherapy. Mol Ther. 2018. https://doi.org/10.1016/j.ymthe.2018.08.002.
Yang H, Zhang Q, Xu M, Wang L, Chen X, Feng Y, et al. CCL2-CCR2 axis recruits tumor associated macrophages to induce immune evasion through PD-1 signaling in esophageal carcinogenesis. Mol Cancer. 2020. https://doi.org/10.1186/s12943-020-01165-x.
Barkal AA, Weiskopf K, Kao KS, Gordon SR, Rosental B, Yiu YY, et al. Engagement of MHC class i by the inhibitory receptor LILRB1 suppresses macrophages and is a target of cancer immunotherapy article. Nat Immunol. 2018. https://doi.org/10.1038/s41590-017-0004-z.
Jaynes JM, Sable R, Ronzetti M, Bautista W, Knotts Z, Abisoye-Ogunniyan A, et al. Mannose receptor (CD206) activation in tumor-associated macrophages enhances adaptive and innate antitumor immune responses. Sci Transl Med. 2020. https://doi.org/10.1126/scitranslmed.aax6337.
Zhou Y, Fei M, Zhang G, Liang WC, Lin WY, Wu Y, et al. Blockade of the phagocytic receptor MerTK on tumor-associated macrophages enhances P2X7R-dependent STING activation by tumor-derived cGAMP. Immunity. 2020. https://doi.org/10.1016/j.immuni.2020.01.014.
Xu C, Ji X, Zhou Y, Cheng Y, Guo D, Li Q, et al. Slimming and reinvigorating tumor-associated dendritic cells with hierarchical lipid rewiring nanoparticles. Adv Mater. 2023. https://doi.org/10.1002/adma.202211415.
Salemme V, Centonze G, Cavallo F, Defilippi P, Conti L. The crosstalk between tumor cells and the immune microenvironment in breast cancer: implications for immunotherapy. Front Oncol. 2021. https://doi.org/10.3389/fonc.2021.610303.
Huang L, Yang Q, Chen H, Wang Z, Liu Q, Ai S. Tollip promotes hepatocellular carcinoma progression via PI3K/AKT pathway. Open Med (Poland). 2022. https://doi.org/10.1515/med-2022-0453.
Zhang Y, Lee C, Geng S, Li L. Enhanced tumor immune surveillance through neutrophil reprogramming due to Tollip deficiency. JCI Insight. 2019. https://doi.org/10.1172/jci.insight.122939.
Begka C, Pattaroni C, Mooser C, Nancey S, McCoy KD, Velin D, et al. Toll-interacting protein regulates immune cell infiltration and promotes colitis-associated cancer. iScience. 2020. https://doi.org/10.1016/j.isci.2020.100891.
Jiang Y, Kong D, Miao X, Yu X, Wu Z, Liu H, et al. Anti-cytokine therapy and small molecule agents for the treatment of inflammatory bowel disease. Eur Cytokine Netw. 2021. https://doi.org/10.1684/ecn.2021.0472.
Maini RN, Taylor PC. Anti-cytokine therapy for rheumatoid arthritis. Annu Rev Med. 2000. https://doi.org/10.1146/annurev.med.51.1.207.
Thompson C, Davies R, Choy E. Anti cytokine therapy in chronic inflammatory arthritis. Cytokine. 2016. https://doi.org/10.1016/j.cyto.2016.07.015.
Heere-Ress E, Boehm J, Thallinger C, Hoeller C, Wacheck V, Birner P, et al. Thalidomide enhances the anti-tumor activity of standard chemotherapy in a human melanoma xenotransplatation model. J Invest Dermatol. 2005. https://doi.org/10.1111/j.0022-202X.2005.23830.x.
Skórka K, Bhattacharya N, Własiuk P, Kowal M, Mertens D, Dmoszyńska A, et al. Thalidomide regulation of NF-κB proteins limits tregs activity in chronic lymphocytic leukemia. Adv Clin Exp Med. 2014. https://doi.org/10.17219/acem/37018.
de Souza CM, de Carvalho LF, da Silva Vieira T, Araújo AC, Lopes MT, et al. Thalidomide attenuates mammary cancer associated-inflammation, angiogenesis and tumor growth in mice. Biomed Pharmacother. 2012. https://doi.org/10.1016/j.biopha.2012.04.005.
Politi PM. [Thalidomide. Clinical trials in cancer] Talidomida. Ensayos clinicos en cancer. Medicina. 2000;60(Suppl 2):61–5.
Chulpanova DS, Kitaeva KV, Green AR, Rizvanov AA, Solovyeva VV. Molecular aspects and future perspectives of cytokine-based anti-cancer immunotherapy. Front Cell Dev Biol. 2020. https://doi.org/10.3389/fcell.2020.00402.
Zhang J, Veeramachaneni N. Targeting interleukin-1β and inflammation in lung cancer. Biomark Res. 2022. https://doi.org/10.1186/s40364-021-00341-5.
Mortara L, Balza E, Bruno A, Poggi A, Orecchia P, Carnemolla B. Anti-cancer therapies employing IL-2 cytokine tumor targeting: contribution of innate, adaptive and immunosuppressive cells in the anti-tumor efficacy. Front Immunol. 2018. https://doi.org/10.3389/fimmu.2018.02905.
Vidal-Vanaclocha F, Fantuzzi G, Mendoza L, Fuentes AM, Anasagasti MJ, Martin J, et al. IL-18 regulates IL-1β-dependent hepatic melanoma metastasis via vascular cell adhesion molecule-1. Proc Natl Acad Sci USA. 2000. https://doi.org/10.1073/pnas.97.2.734.
Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004. https://doi.org/10.1038/nrc1388.
Aldinucci D, Borghese C, Casagrande N. The ccl5/ccr5 axis in cancer progression. Cancers (Basel). 2020. https://doi.org/10.3390/cancers12071765.
Kraus S, Kolman T, Yeung A, Deming D. Chemokine receptor antagonists: role in oncology. Curr Oncol Rep. 2021. https://doi.org/10.1007/s11912-021-01117-8.
Kakinuma T, Hwang ST. Chemokines, chemokine receptors, and cancer metastasis. J Leukoc Biol. 2006. https://doi.org/10.1189/jlb.1105633.
•• den Hollander LS, Béquignon OJM, Wang X, van Wezel K, Broekhuis J, Gorostiola González M, et al. Impact of cancer-associated mutations in CC chemokine receptor 2 on receptor function and antagonism. Biochem Pharmacol. 2023; https://doi.org/10.1016/j.bcp.2022.115399. In this study, the mutations in chemokine receptor CCR2 were analyzed, which is of quite important for the design and subsequent effectiveness of the chemokine antagonists. This paper was integral to the current study.
Nkandeu DS, Basson C, Joubert AM, Serem JC, Bipath P, Nyakudya T, et al. The involvement of a chemokine receptor antagonist CTCE-9908 and kynurenine metabolites in cancer development. Cell Biochem Funct. 2022. https://doi.org/10.1002/cbf.3731.
Author information
Authors and Affiliations
Contributions
A.B.D conceptualized the topic, reviewed the literature and wrote the manuscript. The author approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The author does not declare any conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Demir, A.B. Tumor Promoting Inflammation. Curr Mol Bio Rep 9, 21–32 (2023). https://doi.org/10.1007/s40610-023-00153-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40610-023-00153-6