Abstract
Background
Due to the ongoing Coronavirus disease 2019 (COVID-19) pandemic, interest has arisen to realize the relationship between telomere length (TL) and influenza and pneumonia mortality.
Aim
Our study attempted to investigate this correlation by analyzing information gathered from the National Health and Nutrition Examination Survey (NHANES) 1999–2002.
Methods
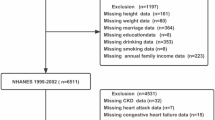
A total of 7229 participants were involved in the conducted research. We utilized Cox proportional risk model analysis to determine the hazard ratio (HR) and 95% confidence interval (CI) for TL and influenza and pneumonia mortality.
Results
During the average follow-up time of 204.10 ± 51.26 months, 33 (0.45%) participants died from influenza and pneumonia. After adjusting for multiple variables, shorter TL was associated with higher influenza–pneumonia mortality. In subgroup analyses stratified by sex, men exhibited stronger associations with influenza–pneumonia mortality than women (Model 1: HRmale: 0.014 vs HRfemale: 0.054; Model 2: HRmale: 0.082 vs HRfemale: 0.890; Model 3: HRmale: 0.072 vs HRfemale: 0.776). For subgroup analyses by visceral adiposity index (VAI), all statistically significant (P < 0.05) models displayed an inverse relationship between TL and influenza and pneumonia mortality.
Conclusions
Our research provides further proof for the connection between shorter telomeres and higher influenza–pneumonia mortality. Larger prospective researches are essential to support our results and explain the underlying mechanisms.

Similar content being viewed by others
Data availability
All materials utilized to conduct this research are openly posted and accessible through references within the manuscript.
References
Jackson ML, Phillips CH, Benoit J et al (2018) Burden of medically attended influenza infection and cases averted by vaccination—United States, 2013/14 through 2015/16 influenza seasons. Vaccine 36:467–472. https://doi.org/10.1016/j.vaccine.2017.12.014
Iuliano AD, Roguski KM, Chang HH et al (2018) Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet 391:1285–1300. https://doi.org/10.1016/S0140-6736(17)33293-2
Yang W, Cummings MJ, Bakamutumaho B et al (2018) Dynamics of influenza in tropical Africa: temperature, humidity, and co-circulating (sub)types. Influenza Other Respir Viruses 12:446–456. https://doi.org/10.1111/irv.12556
Kochanek KD, Murphy SL, Xu J et al (2019) Deaths: final data for 2017. Natl Vital Stat Rep 68:1–77
Kody S, Cline A, Pereira FA (2022) Dermatological trends in emergency medicine. J Dermatolog Treat 33:1746–1748. https://doi.org/10.1080/09546634.2020.1853025
Jain S, Kamimoto L, Bramley AM et al (2009) Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med 361:1935–1944. https://doi.org/10.1056/NEJMoa0906695
Gao H-N, Lu H-Z, Cao B et al (2013) Clinical findings in 111 cases of influenza A (H7N9) virus infection. N Engl J Med 368:2277–2285. https://doi.org/10.1056/NEJMoa1305584
Hariri LP, North CM, Shih AR et al (2021) Lung histopathology in coronavirus disease 2019 as compared with severe acute respiratory sydrome and H1N1 Influenza: a systematic review. Chest 159:73–84. https://doi.org/10.1016/j.chest.2020.09.259
Verma AA, Hora T, Jung HY et al (2021) Characteristics and outcomes of hospital admissions for COVID-19 and influenza in the Toronto area. CMAJ 193:E410–E418. https://doi.org/10.1503/cmaj.202795
Cobb NL, Sathe NA, Duan KI et al (2021) Comparison of clinical features and outcomes in critically ill patients hospitalized with COVID-19 versus influenza. Ann Am Thorac Soc 18:632–640. https://doi.org/10.1513/AnnalsATS.202007-805OC
Bernadotte A, Mikhelson VM, Spivak IM (2016) Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging (Albany NY) 8:3–11. https://doi.org/10.18632/aging.100871
Doksani Y (2019) The response to DNA damage at telomeric repeats and its consequences for telomere function. Genes (Basel) 10:318. https://doi.org/10.3390/genes10040318
Vaziri H, Benchimol S (1996) From telomere loss to p53 induction and activation of a DNA-damage pathway at senescence: the telomere loss/DNA damage model of cell aging. Exp Gerontol 31:295–301. https://doi.org/10.1016/0531-5565(95)02025-x
Haycock PC, Heydon EE, Kaptoge S et al (2014) Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ 349:g4227. https://doi.org/10.1136/bmj.g4227
Zhan Y, Song C, Karlsson R et al (2015) Telomere length shortening and alzheimer disease–a mendelian randomization study. JAMA Neurol 72:1202–1203. https://doi.org/10.1001/jamaneurol.2015.1513
Cheng F, Carroll L, Joglekar MV et al (2021) Diabetes, metabolic disease, and telomere length. Lancet Diabetes Endocrinol 9:117–126. https://doi.org/10.1016/S2213-8587(20)30365-X
Xu X, Qu K, Pang Q et al (2016) Association between telomere length and survival in cancer patients: a meta-analysis and review of literature. Front Med 10:191–203. https://doi.org/10.1007/s11684-016-0450-2
De Boer RJ, Noest AJ (1998) T cell renewal rates, telomerase, and telomere length shortening. J Immunol 160:5832–5837
Cohen S, Janicki-Deverts D, Turner RB et al (2013) Association between telomere length and experimentally induced upper respiratory viral infection in healthy adults. JAMA 309:699–705. https://doi.org/10.1001/jama.2013.613
Anderson JJ, Susser E, Arbeev KG et al (2022) Telomere-length dependent T-cell clonal expansion: a model linking ageing to COVID-19 T-cell lymphopenia and mortality. EBioMedicine 78:103978. https://doi.org/10.1016/j.ebiom.2022.103978
Needham BL, Adler N, Gregorich S et al (2013) Socioeconomic status, health behavior, and leukocyte telomere length in the National Health and Nutrition Examination Survey, 1999–2002. Soc Sci Med 85:1–8. https://doi.org/10.1016/j.socscimed.2013.02.023
Gong H, Yu Q, Yuan M et al (2022) The relationship between dietary copper intake and telomere length in hypertension. J Nutr Health Aging 26:510–514. https://doi.org/10.1007/s12603-022-1787-7
Amato MC, Giordano C (2014) Visceral adiposity index: an indicator of adipose tissue dysfunction. Int J Endocrinol 2014:730827. https://doi.org/10.1155/2014/730827
Charlson ME, Pompei P, Ales KL et al (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383. https://doi.org/10.1016/0021-9681(87)90171-8
Zhao H, Pan Y, Wang C et al (2021) The effects of metal exposures on Charlson comorbidity index using zero-inflated negative binomial regression model: NHANES 2011–2016. Biol Trace Elem Res 199:2104–2111. https://doi.org/10.1007/s12011-020-02331-4
Arnett DK, Blumenthal RS, Albert MA et al (2019) 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 140:e563–e595. https://doi.org/10.1161/CIR.0000000000000677
Wang Q, Zhan Y, Pedersen NL et al (2018) Telomere Length and All-Cause Mortality: A Meta-analysis. Ageing Res Rev 48:11–20. https://doi.org/10.1016/j.arr.2018.09.002
Cawthon RM, Smith KR, O’Brien E et al (2003) Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 361:393–395. https://doi.org/10.1016/S0140-6736(03)12384-7
Schneider CV, Schneider KM, Teumer A et al (2022) Association of telomere length with risk of disease and mortality. JAMA Intern Med 182:291–300. https://doi.org/10.1001/jamainternmed.2021.7804
Fitzpatrick AL, Kronmal RA, Gardner JP et al (2007) Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol 165:14–21. https://doi.org/10.1093/aje/kwj346
Pooley KA, Bojesen SE, Weischer M et al (2013) A genome-wide association scan (GWAS) for mean telomere length within the COGS project: identified loci show little association with hormone-related cancer risk. Hum Mol Genet 22:5056–5064. https://doi.org/10.1093/hmg/ddt355
Codd V, Nelson CP, Albrecht E et al (2013) Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet 45:422–427 (427e1-2). https://doi.org/10.1038/ng.2528
Planas-Cerezales L, Arias-Salgado EG, Buendia-Roldán I et al (2019) Predictive factors and prognostic effect of telomere shortening in pulmonary fibrosis. Respirology 24:146–153. https://doi.org/10.1111/resp.13423
Kelley WJ, Zemans RL, Goldstein DR (2020) Cellular senescence: friend or foe to respiratory viral infections? Eur Respir J 56:2002708. https://doi.org/10.1183/13993003.02708-2020
Ruiz A, Flores-Gonzalez J, Buendia-Roldan I et al (2021) Telomere shortening and its association with cell dysfunction in lung diseases. Int J Mol Sci 23:425. https://doi.org/10.3390/ijms23010425
Chow RD, Majety M, Chen S (2021) The aging transcriptome and cellular landscape of the human lung in relation to SARS-CoV-2. Nat Commun 12:4. https://doi.org/10.1038/s41467-020-20323-9
Álvarez D, Cárdenes N, Sellarés J et al (2017) IPF lung fibroblasts have a senescent phenotype. Am J Physiol Lung Cell Mol Physiol 313:L1164–L1173. https://doi.org/10.1152/ajplung.00220.2017
Jiang C, Liu G, Luckhardt T et al (2017) Serpine 1 induces alveolar type II cell senescence through activating p53–p21-Rb pathway in fibrotic lung disease. Aging Cell 16:1114–1124. https://doi.org/10.1111/acel.12643
Yao C, Guan X, Carraro G et al (2021) Senescence of alveolar type 2 cells drives progressive pulmonary fibrosis. Am J Respir Crit Care Med 203:707–717. https://doi.org/10.1164/rccm.202004-1274OC
Houssaini A, Breau M, Kebe K et al (2018) mTOR pathway activation drives lung cell senescence and emphysema. JCI Insight 3:93203. https://doi.org/10.1172/jci.insight.93203
Lv N, Zhao Y, Liu X et al (2022) Dysfunctional telomeres through mitostress-induced cGAS/STING activation to aggravate immune senescence and viral pneumonia. Aging Cell 21:e13594. https://doi.org/10.1111/acel.13594
Gardner M, Bann D, Wiley L et al (2014) Gender and telomere length: systematic review and meta-analysis. Exp Gerontol 51:15–27. https://doi.org/10.1016/j.exger.2013.12.004
Müezzinler A, Zaineddin AK, Brenner H (2013) A systematic review of leukocyte telomere length and age in adults. Ageing Res Rev 12:509–519. https://doi.org/10.1016/j.arr.2013.01.003
Cutolo M, Montagna P, Brizzolara R et al (2009) Sex hormones modulate the effects of Leflunomide on cytokine production by cultures of differentiated monocyte/macrophages and synovial macrophages from rheumatoid arthritis patients. J Autoimmun 32:254–260. https://doi.org/10.1016/j.jaut.2009.02.016
Giefing-Kröll C, Berger P, Lepperdinger G et al (2015) How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell 14:309–321. https://doi.org/10.1111/acel.12326
de Jong M, Woodward M, Peters SAE (2021) Diabetes and COVID-19-related mortality in women and men in the UK biobank: comparisons with influenza/pneumonia and coronary heart disease. Diabetes Care 44:e22–e24. https://doi.org/10.2337/dc20-2378
Kalil AC, Thomas PG (2019) Influenza virus-related critical illness: pathophysiology and epidemiology. Crit Care 23:258. https://doi.org/10.1186/s13054-019-2539-x
Quandelacy TM, Viboud C, Charu V et al (2014) Age- and sex-related risk factors for influenza-associated mortality in the United States between 1997–2007. Am J Epidemiol 179:156–167. https://doi.org/10.1093/aje/kwt235
Hoffmann J, Otte A, Thiele S et al (2015) Sex differences in H7N9 influenza A virus pathogenesis. Vaccine 33:6949–6954. https://doi.org/10.1016/j.vaccine.2015.08.044
Lorenzo ME, Hodgson A, Robinson DP et al (2011) Antibody responses and cross protection against lethal influenza A viruses differ between the sexes in C57BL/6 mice. Vaccine 29:9246–9255. https://doi.org/10.1016/j.vaccine.2011.09.110
van der Plaat DA, Pereira M, Pesce G et al (2019) Age at menopause and lung function: a Mendelian randomisation study. Eur Respir J 54:1802421. https://doi.org/10.1183/13993003.02421-2018
Fan Y, Guo Y, Zhong J et al (2022) The association between visceral adiposity index and leukocyte telomere length in adults: results from National Health and Nutrition Examination Survey. Aging Clin Exp Res 34:2177–2183. https://doi.org/10.1007/s40520-022-02168-y
Kovacs EJ, Messingham KAN (2002) Influence of alcohol and gender on immune response. Alcohol Res Health 26:257–263
Shen G, Huang J-Y, Huang Y-Q et al (2020) The Relationship between telomere length and cancer mortality: data from the 1999–2002 National Healthy and Nutrition Examination Survey (NHANES). J Nutr Health Aging 24:9–15. https://doi.org/10.1007/s12603-019-1265-z
Weischer M, Bojesen SE, Nordestgaard BG (2014) Telomere shortening unrelated to smoking, body weight, physical activity, and alcohol intake: 4,576 general population individuals with repeat measurements 10 years apart. PLoS Genet 10:e1004191. https://doi.org/10.1371/journal.pgen.1004191
Acknowledgements
Liang Yingshan and Huang Peipei have contributed the same to this work.
Funding
No funds, grants, or other support was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no personal or financial conflicts of interest to declare by the authors.
Ethical approval
Our research was conducted with the approval of the NCHS Research Ethics Review Board in conformity with the Declaration of Helsinki.
Research involving human participants
This article contains no research with human subjects conducted by any of the authors.
Informed consent
Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, Y., Huang, P. Associations of telomere length with risk of mortality from influenza and pneumonia in US adults: a prospective cohort study of NHANES 1999–2002. Aging Clin Exp Res 35, 3115–3125 (2023). https://doi.org/10.1007/s40520-023-02607-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-023-02607-4