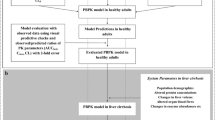
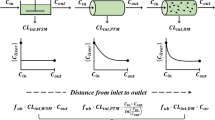
Abstract
Purpose
This review summarizes the development processes of hepatic impairment (HI) PBPK models, examines current challenges, and proposes potential solutions.
Recent Findings
Because hepatic impairment can significantly alter a patient’s physiology, HI PBPK models must consider complex in vivo processes leading to potential changes in PK parameters. Adjustments need to be made to absorption, distribution, metabolism, and elimination parameters. Multiple studies already include changes in levels of CYP enzymes, UGTs, transporters, shunting, and protein binding in their HI models. However, despite recent progress, HI PBPK models face multiple challenges and may overpredict drug exposure with increasing severity of liver dysfunction. Foremost among these challenges is the use of the Child–Pugh scoring system in designing HI PBPK models. Furthermore, most HI PBPK models do not account for changes in certain drug parameters, potentially skewing resulting predictions. Ultimately, limitations with PBPK models can be traced to the scarcity of existing HI PBPK models and clinical data. Filling in this knowledge gap is critical to best support safe drug dosing adjustments for hepatically impaired patients.
Summary
Recent advancements have enhanced the predictive power of HI PBPK models, enabling accurate reflections of clinical trials. However, significant obstacles in developing accurate PK predictions remain, especially for severe impairment.
Similar content being viewed by others
Change history
26 October 2021
A Correction to this paper has been published: https://doi.org/10.1007/s40495-021-00267-4
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Horak J, et al. The effect of different etiologies of hepatic impairment on the pharmacokinetics of gefitinib. Cancer Chemother Pharmacol. 2011;68(6):1485–95.
Verbeeck RK. Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. Eur J Clin Pharmacol. 2008;64(12):1147–61.
•• Kok B, Abraldes JG. Child-Pugh classification: time to abandon? Semin Liver Dis. 2019;39(1):96–103. (This article contains a comprehensive review of the failings of the Child-Pugh scoring system when used to classify disease severity in PBPK models.)
•• Heimbach T, et al. Physiologically-based pharmacokinetic modeling in renal and hepatic impairment populations: a pharmaceutical industry perspective. Clin Pharmacol Ther. 2020;110:297–310. (This paper evaluated the predictive accuracy of current HI PBPK models across a range of compounds.It suggests that for moderate to severe cases of HI, existing models tend to overpredict exposure because they do not account for changes in absorption induced by HI.)
• Morcos PN, et al. Effect of hepatic impairment on the pharmacokinetics of alectinib. J Clin Pharmacol, 2018. 58(12): p. 1618–1628. This article provides a full mechanistic PBPK model of alectinib PK in hepatically impaired patients. The model was successfully used to inform a clinical trial design. (The model was found to have overpredicted the effect of hepatic impairment in patients with moderate to severe liver disease.)
Huang W, et al. Physiologically based pharmacokinetic model of the CYP2D6 probe atomoxetine: extrapolation to special populations and drug-drug interactions. Drug Metab Dispos. 2017;45(11):1156–65.
•• Assessing Changes in Pharmacokinetics of Drugs in Liver Disease, October 8, 2020. This workshop evaluated the impact of hepatic impairment on drug pharmacokinetics. It reviewed the shortcomings of existing hepatic impairment PBPK models. [cited 2021 July 11]; Available from: https://www.fda.gov/drugs/news-events-human-drugs/assessing-changes-pharmacokinetics-drugs-liver-disease-10082020-10082020.
Chang Y, et al. Evaluation of hepatic impairment dosing recommendations in FDA-approved product labels. J Clin Pharmacol. 2013;53(9):962–6.
Food and drug administration, pharmacokinetics in patients with impaired hepatic function: study design, data analysis, and impact on dosing and labeling. 2003 [cited 2021 July 11]; Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pharmacokinetics-patients-impaired-hepatic-function-study-design-data-analysis-and-impact-dosing-and.
Guideline on the evaluation of the pharmacokinetics of medicinal products in patients with impaired hepatic function 2005 [cited 2021 July 11]; Available from: https://www.emaeuropaeu/en/documents/scientific-guideline/guideline-evaluation-pharmacokinetics-medicinal-products-patients-impaired-hepatic-function_enpdf. .
Grimstein M, et al. Physiologically based pharmacokinetic modeling in regulatory science: an update from the U.S. Food and Drug Administration’s Office of Clinical Pharmacology. Journal of pharmaceutical sciences, 2019. 108(1): p. 21–25.
Wang L, et al. Transporter expression in liver tissue from subjects with alcoholic or hepatitis C cirrhosis quantified by targeted quantitative proteomics. Drug Metab Dispos. 2016;44(11):1752–8.
Murray M, et al. Differential effects of hepatic cirrhosis on the intrinsic clearances of sorafenib and imatinib by CYPs in human liver. Eur J Pharm Sci. 2018;114:55–63.
Infante-Rivard C, Esnaola S, Villeneuve JP. Clinical and statistical validity of conventional prognostic factors in predicting short-term survival among cirrhotics. Hepatology. 1987;7(4):660–4.
Chen Y, et al. Simulation of the pharmacokinetics of oseltamivir and its active metabolite in normal populations and patients with hepatic cirrhosis using physiologically based pharmacokinetic modeling. AAPS PharmSciTech. 2020;21(3):98.
Elmeliegy M, et al. Discordance between Child-Pugh and National Cancer Institute Classifications for Hepatic Dysfunction: implications on dosing recommendations for oncology compounds. J Clin Pharmacol. 2021;61(1):105–15.
Johnson TN, et al. A semi-mechanistic model to predict the effects of liver cirrhosis on drug clearance. Clin Pharmacokinet. 2010;49(3):189–206.
Le BH, et al. Oxycodone/naloxone prolonged-release tablets in patients with moderate-to-severe, chronic cancer pain: challenges in the context of hepatic impairment. Asia-Pacific Journal of Clinical Oncology, 2021. n/a(n/a).
Geier A, et al. Side effects of budesonide in liver cirrhosis due to chronic autoimmune hepatitis: influence of hepatic metabolism versus portosystemic shunts on a patient complicated with HCC. World J Gastroenterol. 2003;9(12):2681–5.
Chalasani N, et al. Hepatic and intestinal cytochrome P450 3A activity in cirrhosis: effects of transjugular intrahepatic portosystemic shunts. Hepatology. 2001;34(6):1103–8.
Neal EA, et al. Enhanced bioavailability and decreased clearance of analgesics in patients with cirrhosis. Gastroenterology. 1979;77(1):96–102.
Farthing MJ, et al. Pharmacokinetics of naftopidil, a novel anti-hypertensive drug, in patients with hepatic dysfunction. Postgrad Med J. 1994;70(823):363–6.
Scheers E, et al. Absorption, metabolism, and excretion of oral 14C radiolabeled ibrutinib: an open-label, phase I, single-dose study in healthy men. Drug Metab Dispos. 2015;43(2):289–97.
de Jong J, et al. Single-dose pharmacokinetics of ibrutinib in subjects with varying degrees of hepatic impairment<sup/>. Leuk Lymphoma. 2017;58(1):185–94.
Pomier-Layrargues G, et al. Effect of portacaval shunt on drug disposition in patients with cirrhosis. Gastroenterology. 1986;91(1):163–7.
Li R, Barton HA, Maurer TS. A mechanistic pharmacokinetic model for liver transporter substrates under liver cirrhosis conditions. CPT Pharmacometrics Syst Pharmacol. 2015;4(6):338–49.
Tortorici MA, et al. Influence of mild and moderate hepatic impairment on axitinib pharmacokinetics. Invest New Drugs. 2011;29(6):1370–80.
Reshetnyak VI. Physiological and molecular biochemical mechanisms of bile formation. World J Gastroenterol. 2013;19(42):7341–60.
Turnberg LA, Grahame G. Bile salt secretion in cirrhosis of the liver. Gut. 1970;11(2):126–33.
Burckart GJ, et al. Cyclosporine pharmacokinetic profiles in liver, heart, and kidney transplant patients as determined by high-performance liquid chromatography. Transplant Proc. 1986;18(6 Suppl 5):129–36.
Budha NR, et al. Drug absorption interactions between oral targeted anticancer agents and PPIs: is pH-dependent solubility the Achilles heel of targeted therapy? Clin Pharmacol Ther. 2012;92(2):203–13.
Li J, et al. Differential metabolism of gefitinib and erlotinib by human cytochrome P450 enzymes. Clin Cancer Res. 2007;13(12):3731–7.
O’Bryant CL, et al. An open-label study to describe pharmacokinetic parameters of erlotinib in patients with advanced solid tumors with adequate and moderately impaired hepatic function. Cancer Chemother Pharmacol. 2012;69(3):605–12.
Abou-Alfa GK, et al. Pharmacokinetics and safety of vismodegib in patients with advanced solid malignancies and hepatic impairment. Cancer Chemother Pharmacol. 2017;80(1):29–36.
Sharma MR, et al. Evaluation of food effect on pharmacokinetics of vismodegib in advanced solid tumor patients. Clin Cancer Res. 2013;19(11):3059–67.
Abuhelwa AY, et al. Population pharmacokinetic modeling of itraconazole and hydroxyitraconazole for oral SUBA-itraconazole and sporanox capsule formulations in healthy subjects in fed and fasted states. Antimicrob Agents Chemother. 2015;59(9):5681–96.
Miranda C, et al. Biowaiver or bioequivalence: ambiguity in sildenafil citrate BCS classification. AAPS PharmSciTech. 2018;19(4):1693–8.
Nichols DJ, Muirhead GJ, Harness JA. Pharmacokinetics of sildenafil after single oral doses in healthy male subjects: absolute bioavailability, food effects and dose proportionality. British journal of clinical pharmacology, 2002. 53 Suppl 1(Suppl 1): p. 5S-12S.
Muirhead GJ, et al. The effects of age and renal and hepatic impairment on the pharmacokinetics of sildenafil. Br J Clin Pharmacol, 2002. 53 Suppl 1(Suppl 1): p. 21s-30s.
Horsmans Y, et al. Effects of mild to severe hepatic impairment on the pharmacokinetics of sonidegib: a multicenter, open-label, parallel-group study. Clin Pharmacokinet. 2018;57(3):345–54.
Huang ML, et al. Drug-binding proteins in liver transplant patients. J Clin Pharmacol. 1988;28(6):505–6.
Israili ZH, Dayton PG. Human alpha-1-glycoprotein and its interactions with drugs. Drug Metab Rev. 2001;33(2):161–235.
Application of PBPK modeling to support labelling initiatives: Janssen case study. Presented at Development of Best Practices in Physiologically Based Pharmacokinetic Modeling to Support Clinical Pharmacology Regulatory Decision-Making;. 2019 [cited 2021 July 11]; Available from: https://www.fdagov/media/134175/download.
Williams RL, et al. Naproxen disposition in patients with alcoholic cirrhosis. Eur J Clin Pharmacol. 1984;27(3):291–6.
Orlando R, et al. Irreversible CYP3A inhibition accompanied by plasma protein-binding displacement: a comparative analysis in subjects with normal and impaired liver function. Clin Pharmacol Ther. 2009;85(3):319–26.
Adiwijaya, B., et al. The effect of mild and moderate hepatic impairment on telaprevir pharmacokinetics. 2011. 6th International Workshop on the Clinical Pharmacology of Hepatitis Therapy, Cambridge, MA http://www.regist2virology-educationcom/2011/6HEPPK/docs/05_Gargpdf. 2011 [cited 2021 July 11]; Available from: http://www.regist2virology-educationcom/2011/6HEPPK/docs/05_Gargpdf.
Kiang TK, Wilby KJ, Ensom MH. Telaprevir: clinical pharmacokinetics, pharmacodynamics, and drug-drug interactions. Clin Pharmacokinet. 2013;52(7):487–510.
Willmann S, et al. Applications of physiologically based pharmacokinetic modeling of rivaroxaban—renal and hepatic impairment and drug-drug interaction potential. J Clin Pharmacol. 2021;61(5):656–65.
Gonzalez D, et al. The effect of critical illness on drug distribution. Curr Pharm Biotechnol. 2011;12(12):2030–6.
Gill MA, Kern JW. Altered gentamicin distribution in ascitic patients. Am J Hosp Pharm. 1979;36(12):1704–6.
Blaschke TF. Protein binding and kinetics of drugs in liver diseases. Clin Pharmacokinet. 1977;2(1):32–44.
Bϋdingen F, et al. Relevance of liver failure for anti-infective agents: from pharmacokinetic alterations to dosage adjustments. Ther Adv Infect Dis. 2014;2(1):17–42.
Wang Y, et al. Physiologically-based pharmacokinetic (PBPK) modeling and simulation of pharmacokinetics of hepatitis C NS5A protein inhibitors in non-cirrhotic hepatitis C virus (HCV) patients and patients with hepatic impairment. 2020 [cited 2021 July 17]; Available from: https://ascpt.onlinelibrary.wiley.com/doi/https://doi.org/10.1002/cpt.1732.
Fisher CD, et al. Hepatic cytochrome P450 enzyme alterations in humans with progressive stages of nonalcoholic fatty liver disease. Drug Metab Dispos. 2009;37(10):2087–94.
Chalon SA, et al. Effect of hepatic impairment on the pharmacokinetics of atomoxetine and its metabolites. Clin Pharmacol Ther. 2003;73(3):178–91.
Frye RF, et al. Liver disease selectively modulates cytochrome P450–mediated metabolism. Clin Pharmacol Ther. 2006;80(3):235–45.
Garattini E, Terao M. The role of aldehyde oxidase in drug metabolism. Expert Opin Drug Metab Toxicol. 2012;8(4):487–503.
De Sousa Mendes M, et al. A laboratory-specific scaling factor to predict the in vivo human clearance of aldehyde oxidase substrates. Drug Metab Dispos. 2020;48(11):1231–8.
Dalvie D, Di L. Aldehyde oxidase and its role as a drug metabolizing enzyme. Pharmacol Ther. 2019;201:137–80.
Glaenzel U, et al. Absorption, distribution, metabolism, and excretion of capmatinib (INC280) in healthy male volunteers and in vitro aldehyde oxidase phenotyping of the major metabolite. Drug Metab Dispos. 2020;48(10):873–85.
Jones JP, Korzekwa KR. Predicting intrinsic clearance for drugs and drug candidates metabolized by aldehyde oxidase. Mol Pharm. 2013;10(4):1262–8.
Rowland A, Miners JO, Mackenzie PI. The UDP-glucuronosyltransferases: their role in drug metabolism and detoxification. Int J Biochem Cell Biol. 2013;45(6):1121–32.
Bosilkovska M, et al. Analgesics in patients with hepatic impairment: pharmacology and clinical implications. Drugs. 2012;72(12):1645–69.
Prasad B, et al. Abundance of phase 1 and 2 drug-metabolizing enzymes in alcoholic and hepatitis C cirrhotic livers: a quantitative targeted proteomics study. Drug Metab Dispos. 2018;46(7):943–52.
Kasichayanula S, et al. Influence of hepatic impairment on the pharmacokinetics and safety profile of dapagliflozin: an open-label, parallel-group, single-dose study. Clin Ther. 2011;33(11):1798–808.
Hirano M, et al. Effect of hepatic impairment on the pharmacokinetics of pradigastat, a diacylglycerol acyltransferase 1 (DGAT1) inhibitor. Clin Pharmacokinet. 2015;54(7):761–70.
Upthagrove A, et al. Pradigastat disposition in humans: in vivo and in vitro investigations. Xenobiotica. 2017;47(12):1077–89.
Zhang W, et al. The effect of moderate hepatic impairment on the pharmacokinetics of ipragliflozin, a novel sodium glucose co-transporter 2 (SGLT2) inhibitor. Clin Drug Investig. 2013;33(7):489–96.
Debinski HS, et al. Localization of uridine 5’-diphosphate-glucuronosyltransferase in human liver injury. Gastroenterology. 1995;108(5):1464–9.
Almazroo OA, Miah MK, Venkataramanan R. Drug metabolism in the liver. Clin Liver Dis. 2017;21(1):1–20.
Benet LZ. Predicting drug disposition via application of a biopharmaceutics drug disposition classification system. Basic Clin Pharmacol Toxicol. 2010;106(3):162–7.
Vildhede A, et al. Hepatic uptake of atorvastatin: influence of variability in transporter expression on uptake clearance and drug-drug interactions. Drug Metab Dispos. 2014;42(7):1210–8.
Drozdzik M, et al. Protein abundance of hepatic drug transporters in patients with different forms of liver damage. Clin Pharmacol Ther. 2020;107(5):1138–48.
Patel M, Taskar KS, Zamek-Gliszczynski MJ. Importance of hepatic transporters in clinical disposition of drugs and their metabolites. J Clin Pharmacol. 2016;56(Suppl 7):S23-39.
Thakkar N, Slizgi JR, Brouwer KLR. Effect of liver disease on hepatic transporter expression and function. J Pharm Sci. 2017;106(9):2282–94.
Zollner G, et al. Adaptive changes in hepatobiliary transporter expression in primary biliary cirrhosis. J Hepatol. 2003;38(6):717–27.
Granneman GR, et al. Pharmacokinetics of temafloxacin in patients with liver impairment. Clin Pharmacokinet. 1992;22(Suppl 1):24–32.
Villeneuve JP, Fortunet-Fouin H, Arsène D. Cimetidine kinetics and dynamics in patients with severe liver disease. Hepatology. 1983;3(6):923–7.
Kubitza D, et al. Effect of hepatic impairment on the pharmacokinetics and pharmacodynamics of a single dose of rivaroxaban, an oral, direct Factor Xa inhibitor. Br J Clin Pharmacol. 2013;76(1):89–98.
Morcos PN, et al. Absorption, distribution, metabolism and excretion (ADME) of the ALK inhibitor alectinib: results from an absolute bioavailability and mass balance study in healthy subjects. Xenobiotica. 2017;47(3):217–29.
Morcos PN, et al. Effect of food and esomeprazole on the pharmacokinetics of alectinib, a highly selective ALK inhibitor, in healthy subjects. Clinical Pharmacology in Drug Development. 2017;6(4):388–97.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pharmacometrics and Quantitative System Pharmacology
Rights and permissions
About this article
Cite this article
Han, A.N., Han, B.R., Zhang, T. et al. Hepatic Impairment Physiologically Based Pharmacokinetic Model Development: Current Challenges. Curr Pharmacol Rep 7, 213–226 (2021). https://doi.org/10.1007/s40495-021-00266-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40495-021-00266-5