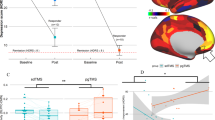
Abstract
Purpose of review
This review uses a dysconnectivity-based lens for understanding schizophrenia pathophysiology. From this vantage recent studies on the clinical utility of transcranial magnetic stimulation in schizophrenia are consolidated, with particular attention to studies reporting resting-state network functional connectivity (FC) changes after treatment.
Recent findings
In schizophrenia, functional connectivity is predominantly decreased across brain regions and functional networks. Specific networks implicated in symptomatology include the default mode network and central executive network. TMS modalities which increase functional connectivity, particularly when targeting the dorsolateral PFC, have shown relative efficacy in treating negative symptoms of schizophrenia. However, post-treatment changes in specific network connectivity vary by study design and by degree of concordance with symptom improvement.
Summary
Generally, increased functional connectivity between brain networks seems to confer some benefit of TMS in schizophrenia, particularly for negative symptoms. Among studies conducted thus far, there has been substantial variation in both target selection and observed post-treatment connectivity changes. While there is convincing evidence for pathological DMN-CEN connectivity in the disease process of schizophrenia, as well as evidence that dlPFC-targeted TMS modulates the DMN-CEN in depression, restoration of this particular network aberrancy by TMS in schizophrenia has not been observed.
Similar content being viewed by others
Data Availability
Not applicable.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
McCutcheon RA, Reis Marques T, Howes OD. Schizophrenia-an overview. JAMA Psychiatry. 2020;77(2):201–10.
Kendler KS, Schaffner KF. The dopamine hypothesis of schizophrenia: an historical and philosophical analysis. Philos Psychiatry Psychol. 2011;18(1):41–63.
Sato M, Chen CC, Akiyama K, Otsuki S. Acute exacerbation of paranoid psychotic state after long-term abstinence in patients with previous methamphetamine psychosis. Biol Psychiatry. 1983;18(4):429–40.
Serper MR, Chou JC, Allen MH, Czobor P, Cancro R. Symptomatic overlap of cocaine intoxication and acute schizophrenia at emergency presentation. Schizophr Bull. 1999;25(2):387–94.
Kalkman HO. Antischizophrenic activity independent of dopamine D2 blockade. Expert Opin Ther Targets. 2002;6(5):571–82.
Mackowick KM, Barr MS, Wing VC, Rabin RA, Ouellet-Plamondon C, George TP. Neurocognitive endophenotypes in schizophrenia: modulation by nicotinic receptor systems. Prog Neuro-Psychopharmacol Biol Psychiatry. 2014;52:79–85.
Li P, Snyder GL, Vanover KE. Dopamine targeting drugs for the treatment of schizophrenia: past, present and future. Curr Top Med Chem. 2016;16(29):3385–403.
de Araujo AN, de Sena EP, de Oliveira IR, Juruena MF. Antipsychotic agents: efficacy and safety in schizophrenia. Drug Healthc Patient Saf. 2012;4:173–80.
Weston-Green K. Antipsychotic drug development: from historical evidence to fresh perspectives. Front Psych. 2022;13:903156.
Kraepelin E, Barclay RM, Robertson GM. Dementia præcox and paraphrenia. Edinburgh: E. & S. Livingstone; 1919. p. 331.
Silverstein ML, Harrow M. Schneiderian first-rank symptoms in schizophrenia. Arch Gen Psychiatry. 1981;38(3):288–93.
Sass LA, Parnas J. Schizophrenia, consciousness, and the self. Schizophr Bull. 2003;29(3):427–44.
Weinberger DR, Wagner RL, Wyatt RJ. Neuropathological studies of schizophrenia: a selective review. Schizophr Bull. 1983;9(2):193–212.
Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122(Pt 4):593–624.
Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. U. S. A. 2003;100(1):253–8.
Andrews-Hanna JR, Smallwood J, Spreng RN. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann. N. Y Acad Sci. 2014;1316(1):29–52.
Mohr HM, Goebel R, Linden DE. Content- and task-specific dissociations of frontal activity during maintenance and manipulation in visual working memory. J. Neurosci. 2006;26(17):4465–71.
Collette F, Van der Linden M. Brain imaging of the central executive component of working memory. Neurosci Biobehav Rev. 2002;26(2):105–25.
Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U. S A. 2008;105(34):12569–74.
Whitfield-Gabrieli S, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U. S. A. 2009;106(4):1279–84.
Chai XJ, et al. Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology. 2011;36(10):2009–17.
Whitfield-Gabrieli S, et al. Understanding marijuana’s effects on functional connectivity of the default mode network in patients with schizophrenia and co-occurring cannabis use disorder: a pilot investigation. Schizophr Res. 2018;194:70–7.
Hermiller MS, VanHaerents S, Raij T, Voss JL. Frequency-specific noninvasive modulation of memory retrieval and its relationship with hippocampal network connectivity. Hippocampus. 2019;29(7):595–609.
Eshel N, et al. Global connectivity and local excitability changes underlie antidepressant effects of repetitive transcranial magnetic stimulation. Neuropsychopharmacology. 2020;45(6):1018–25.
• Tseng PT, et al. Assessment of noninvasive brain stimulation interventions for negative symptoms of schizophrenia: a systematic review and network meta-analysis. JAMA Psychiat. 79(8):770–9. This is a rigorous meta-analysis of neuromodulation trials for schizophrenia, reflecting the current state of the field. Evidence of efficacy to date is strongest for reduction in negative symptoms, with particular successes when interventions target the left dlPFC with excitatory stimulation.
Chopra S, et al. Functional connectivity in antipsychotic-treated and antipsychotic-naive patients with first-episode psychosis and low risk of self-harm or aggression: a secondary analysis of a randomized clinical trial. JAMA Psychiat. 2021;78(9):994–1004.
Chen C, et al. Aberrant functional connectivity of the orbitofrontal cortex is associated with excited symptoms in first-episode drug-naive patients with schizophrenia. Front Psych. 2022;13:922272.
Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164(3):450–7.
Mannell MV, Franco AR, Calhoun VD, Canive JM, Thoma RJ, Mayer AR. Resting state and task-induced deactivation: a methodological comparison in patients with schizophrenia and healthy controls. Hum Brain Mapp. 2010;31(3):424–37.
Salgado-Pineda P, Fakra E, Delaveau P, McKenna PJ, Pomarol-Clotet E, Blin O. Correlated structural and functional brain abnormalities in the default mode network in schizophrenia patients. Schizophr Res. 2011;125(2-3):101–9.
Haatveit B, et al. Reduced load-dependent default mode network deactivation across executive tasks in schizophrenia spectrum disorders. Neuroimage Clin. 2016;12:389–96.
Bolton TAW, et al. Triple network model dynamically revisited: lower salience network state switching in pre-psychosis. Front Physiol. 2020;11:66.
Carhart-Harris RL, et al. Functional connectivity measures after psilocybin inform a novel hypothesis of early psychosis. Schizophr Bull. 2013;39(6):1343–51.
McNabb CB, et al. Functional network dysconnectivity as a biomarker of treatment resistance in schizophrenia. Schizophr Res. 2018;195:160–7.
Nawaz U, Lee I, Beermann A, Eack S, Keshavan M, Brady R. Individual variation in functional brain network topography is linked to schizophrenia symptomatology. Schizophr Bull. 2021;47(1):180–8.
Abualait T, et al. Assessment of cortical plasticity in schizophrenia by transcranial magnetic stimulation. Neural Plast. 2021;2021:5585951.
Baliga SP, Mehta UM. A review of studies leveraging multimodal TMS-fMRI applications in the pathophysiology and treatment of schizophrenia. Front Hum Neurosci. 2021;15:662976.
Jannati A, Oberman LM, Rotenberg A, Pascual-Leone A. Assessing the mechanisms of brain plasticity by transcranial magnetic stimulation. Neuropsychopharmacology. 2023;48(1):191–208.
Liston C, et al. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiat. 2014;76(7):517–26.
Brady RO Jr, et al. Cerebellar-prefrontal network connectivity and negative symptoms in schizophrenia. Am J Psychiat. 2019;176(7):512–20.
Bation R, Magnin C, Poulet E, Mondino M, Brunelin J. Intermittent theta burst stimulation for negative symptoms of schizophrenia-A double-blind, sham-controlled pilot study. NPJ Schizophr. 2021;7(1):10.
Basavaraju R, et al. Intermittent theta burst stimulation of cerebellar vermis enhances fronto-cerebellar resting state functional connectivity in schizophrenia with predominant negative symptoms: a randomized controlled trial. Schizophr Res. 2021;238:108–20.
• Xie Y, et al. Impact of low-frequency repetitive transcranial magnetic stimulation on functional network connectivity in schizophrenia patients with auditory verbal hallucinations. Psychiatry Res. :320, 114974. This study revealed changes in brain network functional connectivity after TMS treatment in schizophrenia, including a specific increase between the Central Executive Network and Auditory network which corresponded to decreased severity of auditory hallucinations.
Lorentzen R, Nguyen TD, McGirr A, Hieronymus F, Ostergaard SD. The efficacy of transcranial magnetic stimulation (TMS) for negative symptoms in schizophrenia: a systematic review and meta-analysis. Schizophrenia (Heidelb). 2022;8(1):35.
Marzouk T, Winkelbeiner S, Azizi H, Malhotra AK, Homan P. Transcranial magnetic stimulation for positive symptoms in schizophrenia: a systematic review. Neuropsychobiology. 2020;79(6):384–96.
Cash RFH, Cocchi L, Lv J, Wu Y, Fitzgerald PB, Zalesky A. Personalized connectivity-guided DLPFC-TMS for depression: advancing computational feasibility, precision and reproducibility. Hum Brain Mapp. 2021;42(13):4155–72.
Funding
Funding support was provided by the University of Maryland/Sheppard Pratt Residency Physician Scientist Training Program (PSTP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
Not applicable
Conflict of Interest
The author declares no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
van der Vaart, A. TMS in Schizophrenia: Potential Mechanistic Insights via Resting-State Network Analyses. Curr Behav Neurosci Rep 10, 58–63 (2023). https://doi.org/10.1007/s40473-023-00260-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40473-023-00260-9