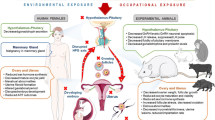
Abstract
Purpose of Review
Epidemiological evidence links certain early-life environmental exposures to adverse health outcomes. However, neonatal biospecimens are difficult to obtain, limiting proper evaluation of the detrimental health effects of environmental exposures during such a critical period of life.
Recent Findings
The availability of archived residual newborn dried blood spots (DBS) from existing newborn screening programs provide a much-needed resource of neonatal biospecimens for epidemiological studies focused on retrospectively assessing the short- and long-term effects of environmental exposures. We discuss emerging omics technologies and biomarkers of exposure/chemical assessments that have recently shown compatibility with the use of DBS.
Summary
Recent developments in the omics technologies are allowing the use of DBS for biomonitoring environmental exposures in community settings, particularly to study the health effects of early life exposures through the use of archived residual newborn DBS. The use of newborn DBS and untargeted omics technologies can dramatically improve the internal exposome assessment. Future studies should continue to validate and standardize the use of DBS as a resource for epidemiological studies.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ostler MW, Porter JH, Buxton OM. Dried blood spot collection of health biomarkers to maximize participation in population studies. J Vis Exp. 2014;(83):e50973.
Grüner N, Stambouli O, Ross RS. Dried blood spots - preparing and processing for use in immunoassays and in molecular techniques. J Vis Exp. 2015;(97):52619.
Tuaillon E, Kania D, Pisoni A, Bollore K, Taieb F, Ontsira Ngoyi EN, et al. Dried blood spot tests for the diagnosis and therapeutic monitoring of HIV and viral hepatitis B and C. Front Microbiol. 2020;11:373.
Blaurock G, Rische H, Rohne K. Development of the blotting paper method in the dried blood reaction for syphilis. Dtsch Gesundheitsw. 1950;5:462–4.
Varshavsky J, Smith A, Wang A, Hom E, Izano M, Huang H, et al. Heightened susceptibility: a review of how pregnancy and chemical exposures influence maternal health. Reprod Toxicol. 2020;92:14–56.
Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect. 2011;119:878–85.
Mitro SD, Johnson T, Zota AR. Cumulative chemical exposures during pregnancy and early development. Curr Environ Health Rep. 2015;2:367–78.
Barker DJP. The origins of the developmental origins theory. J Intern Med. 2007;261:412–7.
Silveira PP, Portella AK, Goldani MZ, Barbieri MA. Developmental origins of health and disease (DOHaD). J Pediatr (Rio J). 2007;83:494–504.
Boekelheide K, Blumberg B, Chapin RE, Cote I, Graziano JH, Janesick A, et al. Predicting later-life outcomes of early-life exposures. Environ Health Perspect. 2012;120:1353–61.
Cohen Hubal EA, Reif DM, Slover R, Mullikin A, Little JC. Children’s environmental health: a systems approach for anticipating impacts from chemicals. Int J Environ Res Public Health. 2020;17:E8337.
De Long NE, Holloway AC. Early-life chemical exposures and risk of metabolic syndrome. Diabetes Metab Syndr Obes. 2017;10:101–9.
Guthrie R, Susi A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics. 1963;32:338–43.
Pitt JJ. Newborn screening. Clin Biochem Rev. 2010;31:57–68.
Bhattacharya K, Wotton T, Wiley V. The evolution of blood-spot newborn screening. Transl Pediatr. 2014;3:63–70.
Pannus P, Claus M, Gonzalez MMP, Ford N, Fransen K. Sensitivity and specificity of dried blood spots for HIV-1 viral load quantification: a laboratory assessment of 3 commercial assays. Medicine. 2016;95:e5475.
Centers for Disease Control and Prevention (CDC). Ten great public health achievements–United States, 2001–2010. MMWR Morb Mortal Wkly Rep. 2011;60:619–23.
Rothwell E, Johnson E, Riches N, Botkin JR. Secondary research uses of residual newborn screening dried bloodspots: a scoping review. Genet Med. 2019;21:1469–75.
De Jesús VR, Mei JV, Cordovado SK, Cuthbert CD. The newborn screening quality assurance program at the centers for disease control and prevention: thirty-five year experience assuring newborn screening laboratory quality. Int J Neonatal Screen. 2015;1:13–26.
Lewis MH, Goldenberg A, Anderson R, Rothwell E, Botkin J. State laws regarding the retention and use of residual newborn screening blood samples. Pediatrics. 2011;127:703–12.
Botkin JR, Rothwell E, Anderson R, Stark L, Goldenberg A, Lewis M, et al. Public attitudes regarding the use of residual newborn screening specimens for research. Pediatrics. 2012;129:231–8.
Tarini BA. Storage and use of residual newborn screening blood spots: a public policy emergency. Genet Med. 2011;13:619–20.
GovTrack.us. H.R. 1281 — 113th congress: newborn screening saves lives reauthorization act of 2014 [Internet]. 2014. Available from: https://www.govtrack.us/congress/bills/113/hr1281. Accessed 21 July 2022
Association of public health laboratories. State Profiles | NewSTEPs [Internet]. APHL. 2022 [cited 2022 Feb 15]. Available from:https://www.newsteps.org/data-resources/state-profiles.
Vrijheid M, Basagaña X, Gonzalez JR, Jaddoe VWV, Jensen G, Keun HC, et al. Advancing tools for human early lifecourse exposome research and translation (ATHLETE). Environ Epidemiol. 2021;5:e166. A longitudinal project from the European Human Exposome Network geared towards developing (1) exposome tools and a European-wide exposome cohort to quantify environmental risk factors on several health outcomes during the first 2 decades of life, (2) develop intervention strategies, and (3) translate the results into policy recommendations and prevention strategies.
Baluch N, Gallant M, Ellis AK. Exposomal research in the context of birth cohorts: what have they taught us? Ann Allergy Asthma Immunol. 2020;125:639–45.
Ni Y, Loftus CT, Szpiro AA, Young MT, Hazlehurst MF, Murphy LE, et al. Associations of pre- and postnatal air pollution exposures with child behavioral problems and cognitive performance: A U.S. multi-cohort study. Environ Health Perspect. 2022;130:067008.
Porpora MG, Piacenti I, Scaramuzzino S, Masciullo L, Rech F, Benedetti PP. Environmental contaminants exposure and preterm birth: a systematic review. Toxics. 2019;7:11.
Krausová M, Braun D, Buerki-Thurnherr T, Gundacker C, Schernhammer E, Wisgrill L, et al. Understanding the chemical exposome during fetal development and early childhood: a review. Annu Rev Pharmacol Toxicol. 2022;63:517–40. An exceptional literature review of the current studies focused on the prenatal chemical exposome that identifies important knowledge gaps and provides recommendations to improve future exposome-scale studies of large cohorts and encourages broader chemical space coverage.
Covaci A, Jorens Ph, Jacquemyn Y, Schepens P. Distribution of PCBs and organochlorine pesticides in umbilical cord and maternal serum. Sci Total Environ. 2002;298:45–53.
Vizcaino E, Grimalt JO, Lopez-Espinosa M-J, Llop S, Rebagliato M, Ballester F. Maternal origin and other determinants of cord serum organochlorine compound concentrations in infants from the general population. Environ Sci Technol. 2010;44:6488–95.
Morello-Frosch R, Cushing LJ, Jesdale BM, Schwartz JM, Guo W, Guo T, et al. Environmental chemicals in an urban population of pregnant women and their newborns from San Francisco. Environ Sci Technol. 2016;50:12464–72.
Neta G, Goldman LR, Barr D, Sjödin A, Apelberg BJ, Witter FR, et al. Distribution and determinants of pesticide mixtures in cord serum using principal component analysis. ACS Publications. 2010;44:5641–8.
Fisher M, Arbuckle TE, Liang CL, LeBlanc A, Gaudreau E, Foster WG, et al. Concentrations of persistent organic pollutants in maternal and cord blood from the maternal-infant research on environmental chemicals (MIREC) cohort study. Environ Health. 2016;15:59.
Hall JM, Lingenfelter P, Adams SL, Lasser D, Hansen JA, Bean MA. Detection of maternal cells in human umbilical cord blood using fluorescence in situ hybridization. Blood. 1995;86:2829–32.
Bauer M, Orescovic I, Schoell WM, Bianchi DW, Pertl B. Detection of maternal deoxyribonucleic acid in umbilical cord plasma by using fluorescent polymerase chain reaction amplification of short tandem repeat sequences. Am J Obstet Gynecol. 2002;186:117–20.
Petit T, Dommergues M, Socié G, Dumez Y, Gluckman E, Brison O. Detection of maternal cells in human fetal blood during the third trimester of pregnancy using allele-specific PCR amplification. Br J Haematol. 1997;98:767–71.
Ma W-L, Gao C, Bell EM, Druschel CM, Caggana M, Aldous KM, et al. Analysis of polychlorinated biphenyls and organochlorine pesticides in archived dried blood spots and its application to track temporal trends of environmental chemicals in newborns. Environ Res. 2014;133:204–10.
Azzopardi PJ, Upshur REG, Luca S, Venkataramanan V, Potter BK, Chakraborty PK, et al. Health-care providers’ perspectives on uncertainty generated by variant forms of newborn screening targets. Genet Med. 2020;22:566–73.
Bailey DB. A window of opportunity for newborn screening. Mol Diagn Ther. 2022;26:253–61.
Friedman JM, Cornel MC, Goldenberg AJ, Lister KJ, Sénécal K, Vears DF. Genomic newborn screening: public health policy considerations and recommendations. BMC Med Genomics. 2017;10:9.
Chan K, Petros M. Simple test, complex system: multifaceted views of newborn screening science, technology, and policy. Glob Pediatr Health. 2019;6:2333794X19894812.
Woerner AC, Gallagher RC, Vockley J, Adhikari AN. The use of whole genome and exome sequencing for newborn screening: challenges and opportunities for population health. Front Pediatr. 2021;9:663752.
Chace DH, Millington DS, Terada N, Kahler SG, Roe CR, Hofman LF. Rapid diagnosis of phenylketonuria by quantitative analysis for phenylalanine and tyrosine in neonatal blood spots by tandem mass spectrometry. Clin Chem. 1993;39:66–71.
Asrani K, Shaw GM, Rine J, Marini NJ. DNA methylome profiling on the Infinium HumanMethylation450 array from limiting quantities of genomic DNA from a single, small archived bloodspot. Genet Test Mol Biomarkers. 2017;21:516–9.
Funk WE, McGee JK, Olshan AF, Ghio AJ. Quantification of arsenic, lead, mercury and cadmium in newborn dried blood spots. Biomarkers. 2013;18:174–7.
Petrick L, Edmands W, Schiffman C, Grigoryan H, Perttula K, Yano Y, et al. An untargeted metabolomics method for archived newborn dried blood spots in epidemiologic studies. Metabolomics. 2017;13:27.
Barr DB, Kannan K, Cui Y, Merrill L, Petrick LM, Meeker JD, et al. The use of dried blood spots for characterizing children’s exposure to organic environmental chemicals. Environ Res. 2021;195:110796. An in-depth narrative review with insightful observations and recommendations regarding the use of dried blood spot matrices for biomonitoring chemical exposures in children’s studies.
Yano Y, Grigoryan H, Schiffman C, Edmands W, Petrick L, Hall K, et al. Untargeted adductomics of Cys34 modifications to human serum albumin in newborn dried blood spots. Anal Bioanal Chem. 2019;411:2351–62.
Gonseth S, Shaw GM, Roy R, Segal MR, Asrani K, Rine J, et al. Epigenomic profiling of newborns with isolated orofacial clefts reveals widespread DNA methylation changes and implicates metastable epiallele regions in disease risk. Epigenetics. 2019;14:198–213.
Stingone JA, Triantafillou S, Larsen A, Kitt JP, Shaw GM, Marsillach J. Interdisciplinary data science to advance environmental health research and improve birth outcomes. Environ Res. 2021;197:111019.
Hasin Y, Seldin M, Lusis A. Multi-omics approaches to disease. Genome Biol. 2017;18:83.
Carlsson H, Rappaport SM, Törnqvist M. Protein adductomics: methodologies for untargeted screening of adducts to serum albumin and hemoglobin in human blood samples. High Throughput. 2019;8.
Cooke MS, Chang Y-J, Chen Y-R, Hu C-W, Chao M-R. Nucleic acid adductomics - the next generation of adductomics towards assessing environmental health risks. Sci Total Environ. 2023;856:159192.
Marsillach J, Costa LG, Furlong CE. Protein adducts as biomarkers of exposure to organophosphorus compounds. Toxicology. 2013;307:46–54.
Marsillach J, Hsieh EJ, Richter RJ, MacCoss MJ, Furlong CE. Proteomic analysis of adducted butyrylcholinesterase for biomonitoring organophosphorus exposures. Chem Biol Interact. 2013;203:85–90.
Yun BH, Guo J, Bellamri M, Turesky RJ. DNA adducts: formation, biological effects, and new biospecimens for mass spectrometric measurements in humans. Mass Spectrom Rev. 2020;39:55–82.
Sabbioni G, Day BW. Quo vadis blood protein adductomics? Arch Toxicol. 2022;96:79–103.
Kumar A, Mhatre S, Godbole S, Jha P, Dikshit R. Optimization of extraction of genomic DNA from archived dried blood spot (DBS): potential application in epidemiological research & bio banking. Gates Open Res. 2018;2:57.
Agrawal P, Katragadda S, Hariharan AK, Raghavendrachar VG, Agarwal A, Dayalu R, et al. Validation of whole genome sequencing from dried blood spots. BMC Med Genomics. 2021;14:110.
Pantazides BG, Quiñones-González J, Rivera Nazario DM, Crow BS, Perez JW, Blake TA, et al. A quantitative method to detect human exposure to sulfur and nitrogen mustards via protein adducts. J Chromatogr B Analyt Technol Biomed Life Sci. 2019;1121:9–17.
Perez JW, Pantazides BG, Watson CM, Thomas JD, Blake TA, Johnson RC. Enhanced stability of blood matrices using a dried sample spot assay to measure human butyrylcholinesterase activity and nerve agent adducts. Anal Chem. 2015;87:5723–9.
Grigoryan H, Edmands W, Lu SS, Yano Y, Regazzoni L, Iavarone AT, et al. Adductomics pipeline for untargeted analysis of modifications to Cys34 of human serum albumin. Anal Chem. 2016;88:10504–12.
Käfferlein HU, Marczynski B, Mensing T, Brüning T. Albumin and hemoglobin adducts of benzo[a]pyrene in humans–analytical methods, exposure assessment, and recommendations for future directions. Crit Rev Toxicol. 2010;40:126–50.
Troester MA, Lindstrom AB, Waidyanatha S, Kupper LL, Rappaport SM. Stability of hemoglobin and albumin adducts of naphthalene oxide, 1,2-naphthoquinone, and 1,4-naphthoquinone. Toxicol Sci. 2002;68:314–21.
Das S, Maras JS, Hussain MdS, Sharma S, David P, Sukriti S, et al. Hyperoxidized albumin modulates neutrophils to induce oxidative stress and inflammation in severe alcoholic hepatitis. Hepatology. 2017;65:631–46.
Ndreu L, Erber LN, Törnqvist M, Tretyakova NY, Karlsson I. Characterizing adduct formation of electrophilic skin allergens with human serum albumin and hemoglobin. Chem Res Toxicol. 2020;33:2623–36.
Peters T. All about albumin. Biochemistry, Genetics, and Medical Applications. [Internet]. San Diego: Academic Press, Inc.; 1995 [cited 2022 Aug 17]. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/food.19970410631
Mock DM, Lankford GL, Widness JA, Burmeister LF, Kahn D, Strauss RG. Measurement of red cell survival using biotin-labeled red cells: validation against 51Cr-labeled red cells. Transfusion. 1999;39:156–62.
Marsillach J, Richter RJ, Kim JH, Stevens RC, MacCoss MJ, Tomazela D, et al. Biomarkers of organophosphorus (OP) exposures in humans. Neurotoxicology. 2011;32:656–60.
Liyasova M, Li B, Schopfer LM, Nachon F, Masson P, Furlong CE, et al. Exposure to tri-o-cresyl phosphate detected in jet airplane passengers. Toxicol Appl Pharmacol. 2011;256:337–47.
Liyasova MS, Schopfer LM, Lockridge O. Cresyl saligenin phosphate makes multiple adducts on free histidine, but does not form an adduct on histidine 438 of human butyrylcholinesterase. Chem Biol Interact. 2013;203:103–7.
Sporty JLS, Lemire SW, Jakubowski EM, Renner JA, Evans RA, Williams RF, et al. Immunomagnetic separation and quantification of butyrylcholinesterase nerve agent adducts in human serum. Anal Chem. 2010;82:6593–600.
Yeowell-O’Connell K, Jin Z, Rappaport SM. Determination of albumin and hemoglobin adducts in workers exposed to styrene and styrene oxide. Cancer Epidemiol Biomarkers Prev. 1996;5:205–15.
Rappaport SM, Waidyanatha S, Qu Q, Shore R, Jin X, Cohen B, et al. Albumin adducts of benzene oxide and 1,4-benzoquinone as measures of human benzene metabolism. Cancer Res. 2002;62:1330–7.
Funk WE, Waidyanatha S, Chaing SH, Rappaport SM. Hemoglobin adducts of benzene oxide in neonatal and adult dried blood spots. Cancer Epidemiol Biomarkers Prev. 2008;17:1896–901.
Funk WE, Montgomery N, Bae Y, Chen J, Chow T, Martinez MP, et al. Human serum albumin Cys34 adducts in newborn dried blood spots: associations with air pollution exposure during pregnancy. Front Public Health. 2021;9:730369. First retrospective study investigating relationships between air pollution exposures during pregnancy by analyzing the albumin adductome of newborn dried blood spots. The authors reported alterations in the albumin adductome of newborns, related to air pollution–induced oxidative stress. The first author pioneered the use of dried blood spots to identify environmental exposures using a protein adductomics approach, back in 2008.
Rappaport SM, Li H, Grigoryan H, Funk WE, Williams ER. Adductomics: characterizing exposures to reactive electrophiles. Toxicol Lett. 2012;213:83–90.
Nunes J, Charneira C, Morello J, Rodrigues J, Pereira SA, Antunes AMM. Mass spectrometry-based methodologies for targeted and untargeted identification of protein covalent adducts (adductomics): current status and challenges. High-Throughput. 2019;8:9.
Yano Y, Schiffman C, Grigoryan H, Hayes J, Edmands W, Petrick L, et al. Untargeted adductomics of newborn dried blood spots identifies modifications to human serum albumin associated with childhood leukemia. Leuk Res. 2020;88:106268. A retrospective study using archived newborn dried blood spots to discover potential risk factors for childhood leukemia via the albumin-Cys34 adductome. Results suggest oxidative stress and lipid peroxidation as potential etiologic factors of childhood T-cell acute lymphoblastic leukemia, and alterations in one-carbon metabolism and epigenetic changes as predictors of childhood acute myeloid leukemia.
Ly A, Buck A, Balluff B, Sun N, Gorzolka K, Feuchtinger A, et al. High-mass-resolution MALDI mass spectrometry imaging of metabolites from formalin-fixed paraffin-embedded tissue. Nat Protoc. 2016;11:1428–43.
Chamberlain CA, Rubio VY, Garrett TJ. Impact of matrix effects and ionization efficiency in non-quantitative untargeted metabolomics. Metabolomics. 2019;15:135.
Maitre L, Robinson O, Martinez D, Toledano MB, Ibarluzea J, Marina LS, et al. Urine metabolic signatures of multiple environmental pollutants in pregnant women: an exposome approach. Environ Sci Technol. 2018;52:13469–80.
Elmsjö A, Haglöf J, Engskog MKR, Erngren I, Nestor M, Arvidsson T, et al. Method selectivity evaluation using the co-feature ratio in LC/MS metabolomics: comparison of HILIC stationary phase performance for the analysis of plasma, urine and cell extracts. J Chromatogr A. 2018;1568:49–56.
Yu M, Tu P, Dolios G, Dassanayake PS, Volk H, Newschaffer C, et al. Tooth biomarkers to characterize the temporal dynamics of the fetal and early-life exposome. Environ Int. 2021;157:106849.
Walker DI, Valvi D, Rothman N, Lan Q, Miller GW, Jones DP. The metabolome: a key measure for exposome research in epidemiology. Curr Epidemiol Rep. 2019;6:93–103.
Barone R, Alaimo S, Messina M, Pulvirenti A, Bastin J, MIMIC-Autism Group, et al. A subset of patients with autism spectrum disorders show a distinctive metabolic profile by dried blood spot analyses. Front Psychiatry. 2018;9:636.
Jing Y, Wu X, Gao P, Fang Z, Wu J, Wang Q, et al. Rapid differentiating colorectal cancer and colorectal polyp using dried blood spot mass spectrometry metabolomic approach. IUBMB Life. 2017;69:347–54.
Bai Q, Peng B, Wu X, Cao Y, Sun X, Hong M, et al. Metabolomic study for essential hypertension patients based on dried blood spot mass spectrometry approach. IUBMB Life. 2018;70:777–85.
Koulman A, Prentice P, Wong MCY, Matthews L, Bond NJ, Eiden M, et al. The development and validation of a fast and robust dried blood spot based lipid profiling method to study infant metabolism. Metabolomics. 2014;10:1018–25.
Ward C, Nallamshetty S, Watrous JD, Acres E, Long T, Mathews IT, et al. Nontargeted mass spectrometry of dried blood spots for interrogation of the human circulating metabolome. J Mass Spectrom. 2021;56:e4772.
Li K, Naviaux JC, Monk JM, Wang L, Naviaux RK. Improved dried blood spot-based metabolomics: a targeted, broad-spectrum, single-injection method. Metabolites. 2020;10:E82.
Tobin NH, Murphy A, Li F, Brummel SS, Taha TE, Saidi F, et al. Comparison of dried blood spot and plasma sampling for untargeted metabolomics. Metabolomics. 2021;17:62.
Dénes J, Szabó E, Robinette SL, Szatmári I, Szőnyi L, Kreuder JG, et al. Metabonomics of newborn screening dried blood spot samples: a novel approach in the screening and diagnostics of inborn errors of metabolism. Anal Chem. 2012;84:10113–20.
Petrick LM, Schiffman C, Edmands WMB, Yano Y, Perttula K, Whitehead T, et al. Metabolomics of neonatal blood spots reveal distinct phenotypes of pediatric acute lymphoblastic leukemia and potential effects of early-life nutrition. Cancer Lett. 2019;452:71–8.
Yu M, Dolios G, Yong-Gonzalez V, Björkqvist O, Colicino E, Halfvarson J, et al. Untargeted metabolomics profiling and hemoglobin normalization for archived newborn dried blood spots from a refrigerated biorepository. J Pharm Biomed Anal. 2020;191:113574. One of the first retrospective untargeted metabolomics study in newborn archived dried blood spots (archived for decades at 4 °C). Importantly, the authors propose the measurement of a hemoglobin sodium lauryl sulfate complex at 540 nm as a normalization factor for robust metabolite measurements in dried blood spots. This is necessary to reduce unwanted variation linked with dried blood spot age and amount of blood.
Petrick LM, Uppal K, Funk WE. Metabolomics and adductomics of newborn bloodspots to retrospectively assess the early-life exposome. Curr Opin Pediatr. 2020;32:300–7. A review about the use of archived newborn dried blood spots for exposomic analyses in retrospective epidemiological studies, particularly of rare and low-frequency pediatric diseases and disorders.
Maitre L, Bustamante M, Hernández-Ferrer C, Thiel D, Lau CH-E, Siskos AP, et al. Multi-omics signatures of the human early life exposome. Nat Commun. 2022;13:7024.
Amine I, Guillien A, Philippat C, Anguita-Ruiz A, Casas M, de Castro M, et al. Environmental exposures in early-life and general health in childhood. Environ Health. 2023;22:53.
Sexton K, Needham LL, Pirkle JL. Human biomonitoring of environmental chemicals. Am Sci. 2004;92:8.
Tan Y-M, Sobus J, Chang D, Tornero-Velez R, Goldsmith M, Pleil J, et al. Reconstructing human exposures using biomarkers and other “clues.” J Toxicol Environ Health B Crit Rev. 2012;15:22–38.
Smolders R, Schramm K-W, Nickmilder M, Schoeters G. Applicability of non-invasively collected matrices for human biomonitoring. Environ Health. 2009;8:8.
Jacobson TA, Kler JS, Bae Y, Chen J, Ladror DT, Iyer R, et al. A state-of-the-science review and guide for measuring environmental exposure biomarkers in dried blood spots. J Expo Sci Environ Epidemiol. 2023;33:505–23. A systematic literature review of available assays for measuring environmental exposure biomarkers in dried blood spots in population-based studies (including newborn sampling). High-performing dried blood spot methods have been developed for measuring several biomarkers of exposure. These show correlation with gold standard venous blood assays. The authors caution about lack of reproducibility and contamination as remaining significant issues for many target analytes in dried blood spot samples.
Sankar P, Cho MK, Condit CM, Hunt LM, Koenig B, Marshall P, et al. Genetic research and health disparities. JAMA. 2004;291:2985–9.
Rappaport SM. Implications of the exposome for exposure science. J Expo Sci Environ Epidemiol. 2011;21:5–9.
Wild CP. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev. 2005;14:1847–50.
Wild CP. The exposome: from concept to utility. Int J Epidemiol. 2012;41:24–32.
Vermeulen R, Schymanski EL, Barabási A-L, Miller GW. The exposome and health: where chemistry meets biology. Science. 2020;367:392–6. An outstanding international collaboration reviewing the exposome concept, and stressing the importance of profiling non-genetic factors associated with disease and health outcomes to identify environmental contributors to health and disease, as comparatively done with the human genome.
van Tongeren M, Cherrie JW. An integrated approach to the exposome. Environ Health Perspect. 2012;120:a103–4.
Turner MC, Nieuwenhuijsen M, Anderson K, Balshaw D, Cui Y, Dunton G, et al. Assessing the exposome with external measures: commentary on the state of the science and research recommendations. Annu Rev Public Health. 2017;38:215–39.
Krassowski M, Das V, Sahu SK, Misra BB. State of the field in multi-omics research: from computational needs to data mining and sharing. Frontiers in Genetics [Internet]. 2020 [cited 2023 Oct 16];11. Available from: https://www.frontiersin.org/articles/10.3389/fgene.2020.610798
Stingone JA, Buck Louis GM, Nakayama SF, Vermeulen RCH, Kwok RK, Cui Y, et al. Toward greater implementation of the exposome research paradigm within environmental epidemiology. Annu Rev Public Health. 2017;38:315–27.
Miller GW. Integrating the exposome into a multi-omic research framework. Exposome. 2021;1:osab002.
Juarez PD, Matthews-Juarez P, Hood DB, Im W, Levine RS, Kilbourne BJ, et al. The public health exposome: a population-based, exposure science approach to health disparities research. Int J Environ Res Public Health. 2014;11:12866–95.
Hejl AM, Adetona O, Diaz-Sanchez D, Carter JD, Commodore AA, Rathbun SL, et al. Inflammatory effects of woodsmoke exposure among wildland firefighters working at prescribed burns at the Savannah River Site. SC J Occup Environ Hyg. 2013;10:173–80.
Adetona AM, Adetona O, Gogal RM, Diaz-Sanchez D, Rathbun SL, Naeher LP. Impact of work task-related acute occupational smoke exposures on select proinflammatory immune parameters in wildland firefighters. J Occup Environ Med. 2017;59:679–90.
Lehner AF, Johnson M, Buchweitz J. Veterinary utility of dried blood spots for analysis of toxic chlorinated hydrocarbons. Toxicol Mech Methods. 2018;28:29–37.
Nyanza EC, Dewey D, Bernier F, Manyama M, Hatfield J, Martin JW. Validation of dried blood spots for maternal biomonitoring of nonessential elements in an artisanal and small-scale gold mining area of Tanzania. Environ Toxicol Chem. 2019;38:1285–93.
Santa-Rios A, Barst BD, Tejeda-Benitez L, Palacios-Torres Y, Baumgartner J, Basu N. Dried blood spots to characterize mercury speciation and exposure in a Colombian artisanal and small-scale gold mining community. Chemosphere. 2021;266:129001.
Barco S, Castagnola E, Moscatelli A, Rudge J, Tripodi G, Cangemi G. Volumetric adsorptive microsampling-liquid chromatography tandem mass spectrometry assay for the simultaneous quantification of four antibiotics in human blood: method development, validation and comparison with dried blood spot. J Pharm Biomed Anal. 2017;145:704–10.
Carling RS, Emmett EC, Moat SJ. Evaluation of volumetric blood collection devices for the measurement of phenylalanine and tyrosine to monitor patients with phenylketonuria. Clin Chim Acta. 2022;535:157–66.
Simeoli R, Cairoli S, Galaverna F, Becilli M, Boccieri E, Antonetti G, et al. Utilization of volumetric absorptive microsampling and dried plasma spot for quantification of anti-fungal triazole agents in pediatric patients by using liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal. 2023;236:115688.
Rodríguez-Saldaña V, Fobil J, Basu N. Lead (Pb) exposure assessment in dried blood spots using Total Reflection X-Ray Fluorescence (TXRF). Environ Res. 2021;198:110444.
Jobst KJ, Arora A, Pollitt KG, Sled JG. Dried blood spots for the identification of bio-accumulating organic compounds: current challenges and future perspectives. Curr Opin Environ Sci Health. 2020;15:66–73. A literature review of studies that analyze chemical pollutants using dried blood spots. The authors highlight the need for continued research to improve sensitivity in analytical chemistry, and for the transition to non-targeted screening approaches in studies using dried blood spots.
Malsagova K, Kopylov A, Stepanov A, Butkova T, Izotov A, Kaysheva A. Dried blood spot in laboratory: directions and prospects. Diagnostics (Basel). 2020;10:248. An exhaustive literature review about dried blood spot technology and potential uses in biomedical research. The authors call for the development of a clear, science-based, legally-supported and coordinated policy for the proper integration of dried blood spot technology in epidemiological studies.
Antunes MV, Charão MF, Linden R. Dried blood spots analysis with mass spectrometry: potentials and pitfalls in therapeutic drug monitoring. Clin Biochem. 2016;49:1035–46.
Winter T, Lange A, Hannemann A, Nauck M, Müller C. Contamination of dried blood spots - an underestimated risk in newborn screening. Clin Chem Lab Med. 2018;56:278–84.
Richardson G, Marshall D, Keevil BG. Prediction of haematocrit in dried blood spots from the measurement of haemoglobin using commercially available sodium lauryl sulphate. Ann Clin Biochem. 2018;55:363–7.
Chepyala D, Kuo H-C, Su K-Y, Liao H-W, Wang S-Y, Chepyala SR, et al. Improved dried blood spot-based metabolomics analysis by a postcolumn infused-internal standard assisted liquid chromatography-electrospray ionization mass spectrometry method. Anal Chem. 2019;91:10702–12.
Timmerman P, White S, Cobb Z, de Vries R, Thomas E, van Baar B. Update of the EBF recommendation for the use of DBS in regulated bioanalysis integrating the conclusions from the EBF DBS-microsampling consortium. Bioanalysis. 2013;5:2129–36.
Lewis MH, Scheurer ME, Green RC, McGuire AL. Research results: preserving newborn blood samples. Sci Trans Med. 2012;4:159cm12–159cm12.
Botkin JR, Goldenberg AJ, Rothwell E, Anderson RA, Lewis MH. Retention and research use of residual newborn screening bloodspots. Pediatrics. 2013;131:120–7.
Yeung EH, Louis GB, Lawrence D, Kannan K, McLain AC, Caggana M, et al. Eliciting parental support for the use of newborn blood spots for pediatric research. BMC Med Res Methodol. 2016;16:14.
Yeung EH, Bell EM, Sundaram R, Ghassabian A, Ma W, Kannan K, et al. Examining endocrine disruptors measured in newborn dried blood spots and early childhood growth in a prospective cohort. Obesity (Silver Spring). 2019;27:145–51.
Robinson SL, Zeng X, Guan W, Sundaram R, Mendola P, Putnick DL, et al. Perfluorooctanoic acid (PFOA) or perfluorooctane sulfonate (PFOS) and DNA methylation in newborn dried blood spots in the Upstate KIDS cohort. Environ Res. 2021;194:110668.
Gallego-Paüls M, Hernández-Ferrer C, Bustamante M, Basagaña X, Barrera-Gómez J, Lau C-HE, et al. Variability of multi-omics profiles in a population-based child cohort. BMC Med. 2021;19:166.
Misra BB, Langefeld CD, Olivier M, Cox LA. Integrated omics: tools, advances, and future approaches. J Mol Endocrinol. 2018;JME-18–0055.
Jamnik T, Flasch M, Braun D, Fareed Y, Wasinger D, Seki D, et al. Next-generation biomonitoring of the early-life chemical exposome in neonatal and infant development. Nat Commun. 2022;13:2653.
Funding
This work was supported by a grant from the National Institute of Environmental Health Sciences (P42 ES004696; JMG and JM), and the start-up funds from the Sheldon D. Murphy Endowment (https://deohs.washington.edu/sheldon-d-murphy-endowed-chair; JM). RAD was supported by a Graduate Student Equity & Excellence (GSEE, formerly GO-MAP) Graduate Fellowship. Funding sources did not direct the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Doung, R.A., Garrick, J.M. & Marsillach, J. Advances in the Use of Residual Newborn Dried Blood Spots Within Environmental Epidemiology. Curr Epidemiol Rep 10, 264–274 (2023). https://doi.org/10.1007/s40471-023-00338-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40471-023-00338-8