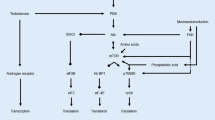
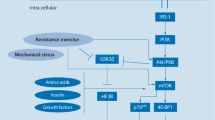
Abstract
Maintaining skeletal muscle mass and function is critical for disease prevention, mobility and quality of life, and whole-body metabolism. Resistance exercise is known to be a major regulator for promoting muscle protein synthesis and muscle mass accretion. Manipulation of exercise intensity, volume, and rest elicit specific muscular adaptations that can maximize the magnitude of muscle growth. The stimulus of muscle contraction that occurs during differing intensities of resistance exercise results in varying biochemical responses regulating the rate of protein synthesis, known as mechanotransduction. At the cellular level, skeletal muscle adaptation appears to be the result of the cumulative effects of transient changes in gene expression following acute bouts of exercise. Thus, maximizing the resistance exercise-induced anabolic response produces the greatest potential for hypertrophic adaptation with training. The mechanisms involved in converting mechanical signals into the molecular events that control muscle growth are not completely understood; however, skeletal muscle protein synthesis appears to be regulated by the multi-protein phosphorylation cascade, mTORC1 (mammalian/mechanistic target of rapamycin complex 1). The purpose of this review is to examine the physiological response to resistance exercise, with particular emphasis on the endocrine response and intramuscular anabolic signaling through mTORC1. It appears that resistance exercise protocols that maximize muscle fiber recruitment, time-under-tension, and metabolic stress will contribute to maximizing intramuscular anabolic signaling; however, the resistance exercise parameters for maximizing the anabolic response remain unclear.


Similar content being viewed by others
References
Braith RW, Stewart KJ. Resistance exercise training its role in the prevention of cardiovascular disease. Circulation. 2006;113(22):2642–50.
Yanagita M, Shiotsu Y. Role of resistance training for preventing frailty and metabolic syndromes in aged adults. J Phys Fit Sports Med. 2014;3(1):35–42.
Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50(5):889–96.
Peterson MD, Gordon PM. Resistance exercise for the aging adult: clinical implications and prescription guidelines. Am J Med. 2011;124(3):194–8.
Baskin KK, Winders BR, Olson EN. Muscle as a “mediator” of systemic metabolism. Cell Metab. 2015;21(2):237–48.
Goodman CA, Mayhew DL, Hornberger TA. Recent progress toward understanding the molecular mechanisms that regulate skeletal muscle mass. Cell Signal. 2011;23(12):1896–906.
Greenhaff PL, Karagounis L, Peirce N, et al. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol Endocrinol Metab. 2008;295(3):E595–604.
Lüthi J, Howald H, Claassen H, et al. Structural changes in skeletal muscle tissue with heavy-resistance exercise. Int J Sports Med. 1986;7(3):123–7.
Paul AC, Rosenthal N. Different modes of hypertrophy in skeletal muscle fibers. J Cell Biol. 2002;156(4):751–60.
Toigo M, Boutellier U. New fundamental resistance exercise determinants of molecular and cellular muscle adaptations. Eur J Appl Physiol. 2006;97(6):643–63.
Kelley G. Mechanical overload and skeletal muscle fiber hyperplasia: a meta-analysis. J Appl Physiol. 1996;81(4):1584–8.
Phillips SM, Tipton KD, Aarsland A, et al. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol Endocrinol Metab. 1997;36(1):E99.
Yarasheski KE, Zachwieja JJ, Bier DM. Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am J Physiol. 1993;265:E210-E.
MacDougall JD, Gibala MJ, Tarnopolsky MA, et al. The time course for elevated muscle protein synthesis following heavy resistance exercise. Can J Appl Physiol. 1995;20(4):480–6.
Chesley A, MacDougall J, Tarnopolsky M, et al. Changes in human muscle protein synthesis after resistance exercise. J Appl Physiol. 1992;73:1383–8.
Aagaard P, Andersen JL, Dyhre-Poulsen P, et al. A mechanism for increased contractile strength of human pennate muscle in response to strength training: changes in muscle architecture. J Physiol. 2001;534(2):613–23.
Bell G, Syrotuik D, Martin T, et al. Effect of concurrent strength and endurance training on skeletal muscle properties and hormone concentrations in humans. Eur J Appl Physiol. 2000;81(5):418–27.
Seynnes OR, de Boer M, Narici MV. Early skeletal muscle hypertrophy and architectural changes in response to high-intensity resistance training. J Appl Physiol. 2007;102(1):368–73.
McCall G, Byrnes W, Dickinson A, et al. Muscle fiber hypertrophy, hyperplasia, and capillary density in college men after resistance training. J Appl Physiol. 1996;81(5):2004–12.
Adams GR, Bamman MM. Characterization and regulation of mechanical loading-induced compensatory muscle hypertrophy. Compr Physiol. 2012;2(4):2829–70.
Hornberger TA. Mechanotransduction and the regulation of mTORC1 signaling in skeletal muscle. Int J Biochem Cell Biol. 2011;43(9):1267–76.
Coffey VG, Hawley JA. The molecular bases of training adaptation. Sports Med. 2007;37(9):737–63.
Tanimoto M, Ishii N. Effects of low-intensity resistance exercise with slow movement and tonic force generation on muscular function in young men. J Appl Physiol. 2006;100(4):1150–7.
Mitchell CJ, Churchward-Venne TA, West DW, et al. Resistance exercise load does not determine training-mediated hypertrophic gains in young men. J Appl Physiol. 2012;113(1):71–7.
Ogasawara R, Loenneke JP, Thiebaud RS, et al. Low-load bench press training to fatigue results in muscle hypertrophy similar to high-load bench press training. Int J Clin Med. 2013;4(2):114.
Kraemer WJ, Nindl BC, Ratamess NA, et al. Changes in muscle hypertrophy in women with periodized resistance training. Med Sci Sports Exerc. 2004;36(4):697–708.
Popov D, Swirkun D, Netreba A, et al. Hormonal adaptation determines the increase in muscle mass and strength during low-intensity strength training without relaxation. Hum Physiol. 2006;32(5):609–14.
Hisaeda H, Miyagawa K, Kuno S, et al. Influence of two different modes of resistance training in female subjects. Ergonomics. 1996;39(6):842.
Chestnut JL, Docherty D. The effects of 4 and 10 repetition maximum weight-training protocols on neuromuscular adaptations in untrained men. J Strength Cond Res. 1999;13(4):353–9.
Léger B, Cartoni R, Praz M, et al. Akt signalling through GSK-3β, mTOR and Foxo1 is involved in human skeletal muscle hypertrophy and atrophy. J Physiol. 2006;576(3):923–33.
Lamon S, Wallace MA, Léger B, et al. Regulation of stars and its downstream targets suggest a novel pathway involved in human skeletal muscle hypertrophy and atrophy. J Physiol. 2009;587(8):1795–803.
Alegre LM, Aguado X, Rojas-Martín D, et al. Load-controlled moderate and high-intensity resistance training programs provoke similar strength gains in young women. Muscle Nerve. 2015;51(1):92–101.
Tanimoto M, Sanada K, Yamamoto K, et al. Effects of whole-body low-intensity resistance training with slow movement and tonic force generation on muscular size and strength in young men. J Strength Cond Res. 2008;22(6):1926–38.
Holm L, Reitelseder S, Pedersen TG, et al. Changes in muscle size and MHC composition in response to resistance exercise with heavy and light loading intensity. J Appl Physiol. 2008;105(5):1454–61.
Campos GE, Luecke TJ, Wendeln HK, et al. Muscular adaptations in response to three different resistance-training regimens: specificity of repetition maximum training zones. Eur J Appl Physiol. 2002;88(1–2):50–60.
Schuenke MD, Herman JR, Gliders RM, et al. Early-phase muscular adaptations in response to slow-speed versus traditional resistance-training regimens. Eur J Appl Physiol. 2012;112(10):3585–95.
Masuda K, Choi JY, Shimojo H, et al. Maintenance of myoglobin concentration in human skeletal muscle after heavy resistance training. Eur J Appl Physiol Occup Physiol. 1999;79(4):347–52.
Choi J, Takahashi H, Itai Y, et al. The difference between effects of “power-up type” and “bulk-up type” strength training exercises-with special reference to muscle cross-sectional area, muscular strength, anaerobic power and anaerobic endurance. Jpn J Phys Fit Sports Med. 1998;47(1):119–29.
Krieger JW. Single vs. multiple sets of resistance exercise for muscle hypertrophy: a meta-analysis. J Strength Cond Res. 2010;24(4):1150–9.
Kim PL, Staron RS, Phillips SM. Fasted-state skeletal muscle protein synthesis after resistance exercise is altered with training. J Physiol. 2005;568(1):283–90.
Phillips SM, Tipton K, Ferrando AA, et al. Resistance training reduces the acute exercise-induced increase in muscle protein turnover. Am J Physiol Endocrinol Metab. 1999;276(1):E118–24.
Tang JE, Perco JG, Moore DR, et al. Resistance training alters the response of fed state mixed muscle protein synthesis in young men. Am J Phys Reg Integr Compar Physiol. 2008;294(1):R172–8.
Coffey V, Zhong Z, Shield A, et al. Early signaling responses to divergent exercise stimuli in skeletal muscle from well-trained humans. FASEB J. 2006;20(1):190–2.
Nader GA, von Walden F, Liu C, et al. Resistance exercise training modulates acute gene expression during human skeletal muscle hypertrophy. J Appl Physiol. 2014;116(6):693–702.
Gonzalez AM, Hoffman JR, Townsend JR, et al. Association between myosin heavy chain protein isoforms and intramuscular anabolic signaling following resistance exercise in trained men. Physiol Rep. 2015;3(1):e12268.
Schoenfeld BJ, Ratamess NA, Peterson MD, et al. Effects of different volume-equated resistance training loading strategies on muscular adaptations in well-trained men. J Strength Cond Res. 2014;28(10):2909–18.
Schoenfeld BJ, Peterson MD, Ogborn D, et al. Effects of low-versus high-load resistance training on muscle strength and hypertrophy in well-trained men. J Strength Cond Res. 2015;29(10):2954–63.
Gehlert S, Suhr F, Gutsche K, et al. High force development augments skeletal muscle signalling in resistance exercise modes equalized for time under tension. Pflügers Arch. 2014;467(6):1343–56.
Burd NA, Andrews RJ, West DW, et al. Muscle time under tension during resistance exercise stimulates differential muscle protein sub-fractional synthetic responses in men. J Physiol. 2012;590(2):351–62.
Popov DV, Lysenko EA, Bachinin AV, et al. Influence of resistance exercise intensity and metabolic stress on anabolic signaling and expression of myogenic genes in skeletal muscle. Muscle Nerve. 2015;51(3):434–42.
Timmons JA. Variability in training-induced skeletal muscle adaptation. J Appl Physiol. 2011;110(3):846–53.
Bamman MM, Petrella JK, Kim J, et al. Cluster analysis tests the importance of myogenic gene expression during myofiber hypertrophy in humans. J Appl Physiol. 2007;102(6):2232–9.
Hubal MJ, Gordish-Dressman H, Thompson PD, et al. Variability in muscle size and strength gain after unilateral resistance training. Med Sci Sports Exerc. 2005;37(6):964–72.
Mitchell CJ, Churchward-Venne TA, Bellamy L, et al. Muscular and systemic correlates of resistance training-induced muscle hypertrophy. PLoS One. 2013;8(10):e78636.
Koopman R, Zorenc AH, Gransier RJ, et al. Increase in S6K1 phosphorylation in human skeletal muscle following resistance exercise occurs mainly in type II muscle fibers. Am J Physiol Endocrinol Metab. 2006;290(6):E1245–52.
Schoenfeld BJ, Ratamess NA, Peterson MD, et al. Influence of resistance training frequency on muscular adaptations in well-trained men. J Strength Cond Res. 2015;29(7):1821–9.
McDonagh M, Davies C. Adaptive response of mammalian skeletal muscle to exercise with high loads. Eur J Appl Physiol Occup Physiol. 1984;52(2):139–55.
Wernbom M, Augustsson J, Thomeé R. The influence of frequency, intensity, volume and mode of strength training on whole muscle cross-sectional area in humans. Sports Med. 2007;37(3):225–64.
Fry AC. The role of resistance exercise intensity on muscle fibre adaptations. Sports Med. 2004;34(10):663–79.
Burd NA, West DW, Staples AW, et al. Low-load high volume resistance exercise stimulates muscle protein synthesis more than high-load low volume resistance exercise in young men. PLoS One. 2010;5(8):e12033.
Crewther B, Cronin J, Keogh J, et al. The salivary testosterone and cortisol response to three loading schemes. J Strength Cond Res. 2008;22(1):250–5.
Hakkinen K, Pakarinen A. Acute hormonal responses to two different fatiguing heavy-resistance protocols in male athletes. J Appl Physiol. 1993;74(2):882–7.
Linnamo V, Pakarinen A, Komi PV, et al. Acute hormonal responses to submaximal and maximal heavy resistance and explosive exercises in men and women. J Strength Cond Res. 2005;19(3):566–71.
McCaulley GO, McBride JM, Cormie P, et al. Acute hormonal and neuromuscular responses to hypertrophy, strength and power type resistance exercise. Eur J Appl Physiol. 2009;105(5):695–704.
Smilios I, Pilianidis T, Karamouzis M, et al. Hormonal responses after various resistance exercise protocols. Med Sci Sports Exerc. 2003;35(4):644–54.
Uchida MC, Crewther BT, Ugrinowitsch C, et al. Hormonal responses to different resistance exercise schemes of similar total volume. J Strength Cond Res. 2009;23(7):2003–8.
Kraemer WJ, Marchitelli L, Gordon SE, et al. Hormonal and growth factor responses to heavy resistance exercise protocols. J Appl Physiol. 1990;69(4):1442–50.
West DW, Burd NA, Tang JE, et al. Elevations in ostensibly anabolic hormones with resistance exercise enhance neither training-induced muscle hypertrophy nor strength of the elbow flexors. J Appl Physiol. 2010;108(1):60–7.
West DW, Burd NA, Staples AW, et al. Human exercise-mediated skeletal muscle hypertrophy is an intrinsic process. Int J Biochem Cell Biol. 2010;42(9):1371–5.
West DW, Kujbida GW, Moore DR, et al. Resistance exercise-induced increases in putative anabolic hormones do not enhance muscle protein synthesis or intracellular signalling in young men. J Physiol. 2009;587(21):5239–47.
Goldberg AL, Etlinger JD, Goldspink DF, et al. Mechanism of work-induced hypertrophy of skeletal muscle. Med Sci Sports. 1974;7(3):185–98.
Brian M, Bilgen E, Diane CF. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem J. 2012;441(1):1–21.
Drummond MJ, Fry CS, Glynn EL, et al. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol. 2009;587(7):1535–46.
Hornberger TA, Sukhija KB, Chien S. Regulation of mTOR by mechanically induced signaling events in skeletal muscle. Cell Cycle. 2006;5(13):1391–6.
Goodman CA. The role of mTORC1 in regulating protein synthesis and skeletal muscle mass in response to various mechanical stimuli. Rev Physiol Biochem Pharmacol. 2014;166:43–95.
Bodine SC, Stitt TN, Gonzalez M, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3(11):1014–9.
Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–93.
Anthony JC, Yoshizawa F, Anthony TG, et al. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000;130(10):2413–9.
Kubica N, Bolster DR, Farrell PA, et al. Resistance exercise increases muscle protein synthesis and translation of eukaryotic initiation factor 2bϵ mRNA in a mammalian target of rapamycin-dependent manner. J Biol Chem. 2005;280(9):7570–80.
Gundermann DM, Walker DK, Reidy PT, et al. Activation of mTORC1 signaling and protein synthesis in human muscle following blood flow restriction exercise is inhibited by rapamycin. Am J Physiol Endocrinol Metab. 2014;306(10):E1198–204.
Hornberger TA, McLoughlin TJ, Leszczynski JK, et al. Selenoprotein-deficient transgenic mice exhibit enhanced exercise-induced muscle growth. J Nutr. 2003;133(10):3091–7.
Kumar V, Selby A, Rankin D, et al. Age-related differences in the dose–response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol. 2009;587(1):211–7.
Burd NA, Holwerda AM, Selby KC, et al. Resistance exercise volume affects myofibrillar protein synthesis and anabolic signalling molecule phosphorylation in young men. J Physiol. 2010;588(16):3119–30.
Baar K, Esser K. Phosphorylation of p70s6k correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol Cell Physiol. 1999;276(1):C120–7.
Terzis G, Georgiadis G, Stratakos G, et al. Resistance exercise-induced increase in muscle mass correlates with p70s6 kinase phosphorylation in human subjects. Eur J Appl Physiol. 2008;102(2):145–52.
Mayhew DL, J-s Kim, Cross JM, et al. Translational signaling responses preceding resistance training-mediated myofiber hypertrophy in young and old humans. J Appl Physiol. 2009;107(5):1655–62.
Goodman CA, Frey JW, Mabrey DM, et al. The role of skeletal muscle mTOR in the regulation of mechanical load-induced growth. J Physiol. 2011;589(22):5485–501.
Marcotte GR, West DW, Baar K. The molecular basis for load-induced skeletal muscle hypertrophy. Calc Tiss Int. 2014;96(3):196–210.
Sato T, Nakashima A, Guo L, et al. Specific activation of mTORC1 by Rheb G-protein in vitro involves enhanced recruitment of its substrate protein. J Biol Chem. 2009;284(19):12783–91.
Tee AR, Manning BD, Roux PP, et al. Tuberous sclerosis complex gene products, tuberin and hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol. 2003;13(15):1259–68.
Menon S, Dibble CC, Talbott G, et al. Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. Cell. 2014;156(4):771–85.
Sandri M. Signaling in muscle atrophy and hypertrophy. Physiology. 2008;23(3):160–70.
Jacobs BL, You J-S, Frey JW, et al. Eccentric contractions increase the phosphorylation of tuberous sclerosis complex-2 (TSC2) and alter the targeting of TSC2 and the mechanistic target of rapamycin to the lysosome. J Physiol. 2013;591(18):4611–20.
Hornberger T, Stuppard R, Conley K, et al. Mechanical stimuli regulate rapamycin-sensitive signalling by a phosphoinositide 3-kinase-, protein kinase B- and growth factor-independent mechanism. Biochem J. 2004;380:795–804.
Deldicque L, Atherton P, Patel R, et al. Effects of resistance exercise with and without creatine supplementation on gene expression and cell signaling in human skeletal muscle. J Appl Physiol. 2008;104(2):371–8.
Deldicque L, Atherton P, Patel R, et al. Decrease in Akt/PKB signalling in human skeletal muscle by resistance exercise. Eur J Appl Physiol. 2008;104(1):57–65.
Hornberger T, Chu W, Mak Y, et al. The role of phospholipase D and phosphatidic acid in the mechanical activation of mTOR signaling in skeletal muscle. Proc Natl Acad Sci. 2006;103(12):4741–6.
You J-S, Lincoln HC, Kim C-R, et al. The role of diacylglycerol kinase ζ and phosphatidic acid in the mechanical activation of mammalian target of rapamycin (mTOR) signaling and skeletal muscle hypertrophy. J Biol Chem. 2014;289(3):1551–63.
Tang W, Yuan J, Chen X, et al. Identification of a novel human lysophosphatidic acid acyltransferase, LPAAT-theta, which activates mTOR pathway. J Biochem Mol Biol. 2006;39(5):626.
Ávila-Flores A, Santos T, Rincón E, et al. Modulation of the mammalian target of rapamycin pathway by diacylglycerol kinase-produced phosphatidic acid. J Biol Chem. 2005;280(11):10091–9.
Fang Y, Vilella-Bach M, Bachmann R, et al. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science. 2001;294(5548):1942–5.
Chen J, Fang Y. A novel pathway regulating the mammalian target of rapamycin (mTOR) signaling. Biochem Pharmacol. 2002;64(7):1071–7.
Sun Y, Fang Y, Yoon M-S, et al. Phospholipase D1 is an effector of Rheb in the mTOR pathway. Proc Natl Acad Sci. 2008;105(24):8286–91.
Wang X, Devaiah SP, Zhang W, et al. Signaling functions of phosphatidic acid. Progr Lipid Res. 2006;45(3):250–78.
Foster DA, Salloum D, Menon D, et al. Phospholipase D and the maintenance of phosphatidic acid levels for regulation of mammalian target of rapamycin (mTOR). J Biol Chem. 2014;289(33):22583–8.
Joy JM, Gundermann DM, Lowery RP, et al. Phosphatidic acid enhances mTOR signaling and resistance exercise induced hypertrophy. Nutr Metab. 2014;11(1):29.
Shepherd P, Withers D, Siddle K. Phosphoinositide 3-kinase: the key switch mechanism in insulin signalling. Biochem J. 1998;333:471–90.
Alessi DR, Cohen P. Mechanism of activation and function of protein kinase B. Curr Opin Gen Dev. 1998;8(1):55–62.
Alessi DR, James SR, Downes CP, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase B. Curr Biol. 1997;7(4):261–9.
Inoki K, Li Y, Zhu T, et al. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4(9):648–57.
Vander Haar E, Lee S-I, Bandhakavi S, et al. Insulin signalling to mTOR mediated by the Akt/PKB substrate pras40. Nat Cell Biol. 2007;9(3):316–23.
Veldhuis JD, Roemmich JN, Richmond EJ, et al. Endocrine control of body composition in infancy, childhood, and puberty. Endocr Rev. 2005;26(1):114–46.
Solomon A, Bouloux P. Modifying muscle mass–the endocrine perspective. J Endocrinol. 2006;191(2):349–60.
Schroeder ET, Villanueva M, West D, et al. Are acute post-resistance exercise increases in testosterone, growth hormone, and IGF-1 necessary to stimulate skeletal muscle anabolism and hypertrophy? Med Sci Sports Exerc. 2013;45(11):2044–51.
McCall GE, Byrnes WC, Fleck SJ, et al. Acute and chronic hormonal responses to resistance training designed to promote muscle hypertrophy. Can J Appl Physiol. 1999;24(1):96–107.
Ahtiainen JP, Pakarinen A, Alen M, et al. Muscle hypertrophy, hormonal adaptations and strength development during strength training in strength-trained and untrained men. Eur J Appl Physiol. 2003;89(6):555–63.
Kraemer WJ, Ratamess NA. Hormonal responses and adaptations to resistance exercise and training. Sports Med. 2005;35(4):339–61.
Wilkinson SB, Tarnopolsky MA, Grant EJ, et al. Hypertrophy with unilateral resistance exercise occurs without increases in endogenous anabolic hormone concentration. Eur J Appl Physiol. 2006;98(6):546–55.
Spiering BA, Kraemer WJ, Anderson JM, et al. Effects of elevated circulating hormones on resistance exercise-induced Akt signaling. Med Sci Sports Exerc. 2008;40(6):1039–48.
Griggs RC, Kingston W, Jozefowicz RF, et al. Effect of testosterone on muscle mass and muscle protein synthesis. J Appl Physiol. 1989;66(1):498–503.
Ferrando AA, Tipton KD, Doyle D, et al. Testosterone injection stimulates net protein synthesis but not tissue amino acid transport. Am J Physiol Endocrinol Metab. 1998;275(5):E864–71.
Bhasin S, Storer TW, Berman N, et al. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335(1):1–7.
Bhasin S, Woodhouse L, Casaburi R, et al. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab. 2001;281(6):E1172–81.
Tenover JS. Effects of testosterone supplementation in the aging male. J Clin Endocrinol Metab. 1992;75(4):1092–8.
Tenover JL. Experience with testosterone replacement in the elderly. Mayo Clin Proc. 2000;75(Suppl): S77–81 (discussion S82).
Morley JE, Perry H, Kaiser F, et al. Effects of testosterone replacement therapy in old hypogonadal males: a preliminary study. J Am Geriatr Soc. 1993;41(2):149–52.
Sih R, Morley JE, Kaiser FE, et al. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab. 1997;82(6):1661–7.
Snyder PJ, Peachey H, Hannoush P, et al. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab. 1999;84(8):2647–53.
Ferrando AA, Sheffield-Moore M, Yeckel CW, et al. Testosterone administration to older men improves muscle function: Molecular and physiological mechanisms. Am J Physiol Endocrinol Metab. 2002;282(3):E601–7.
McGlory C, Phillips SM. Exercise and the regulation of skeletal muscle hypertrophy. Progr Mol Biol Trans Sci. 2015;135:153–73.
Goodman CA, Miu MH, Frey JW, et al. A phosphatidylinositol 3-kinase/protein kinase B-independent activation of mammalian target of rapamycin signaling is sufficient to induce skeletal muscle hypertrophy. Mol Biol Cell. 2010;21(18):3258–68.
Ahtiainen JP, Pakarinen A, Alen M, et al. Short vs. long rest period between the sets in hypertrophic resistance training: Influence on muscle strength, size, and hormonal adaptations in trained men. J Strength Cond Res. 2005;19(3):572–82.
Boroujerdi SS, Rahimi R. Acute GH and IGF-I responses to short vs. long rest period between sets during forced repetitions resistance training system. S Afr J Res Sport Phys Educ Recreat. 2008;30(2):31–8.
Beaven CM, Gill ND, Cook CJ. Salivary testosterone and cortisol responses in professional rugby players after four resistance exercise protocols. J Strength Cond Res. 2008;22(2):426–32.
Goto K, Sato K, Takamatsu K. A single set of low intensity resistance exercise immediately following high intensity resistance exercise stimulates growth hormone secretion in men. J Phys Fit Sports Med. 2003;43(2):243–9.
Kraemer WJ, Aguilera BA, Terada M, et al. Responses of IGF-I to endogenous increases in growth hormone after heavy-resistance exercise. J Appl Physiol. 1995;79(4):1310–5.
Kraemer WJ, Häkkinen K, Newton RU, et al. Effects of heavy-resistance training on hormonal response patterns in younger vs. older men. J Appl Physiol. 1999;87(3):982–92.
Villanueva MG, Villanueva MG, Lane CJ, et al. Influence of rest interval length on acute testosterone and cortisol responses to volume-load–equated total body hypertrophic and strength protocols. J Strength Cond Res. 2012;26(10):2755–64.
Raastad T, Bjøro T, Hallen J. Hormonal responses to high-and moderate-intensity strength exercise. Eur J Appl Physiol. 2000;82(1–2):121–8.
West DW, Phillips SM. Associations of exercise-induced hormone profiles and gains in strength and hypertrophy in a large cohort after weight training. Eur J Appl Physiol. 2012;112(7):2693–702.
Mitchell CJ, Churchward-Venne TA, Parise G, et al. Acute post-exercise myofibrillar protein synthesis is not correlated with resistance training-induced muscle hypertrophy in young men. PLoS One. 2014;9(2):e89431.
Sheffield-Moore M. Androgens and the control of skeletal muscle protein synthesis. Ann Med. 2000;32(3):181–6.
Willoughby DS, Taylor L. Effects of sequential bouts of resistance exercise on androgen receptor expression. Med Sci Sports Exerc. 2004;36(9):1499–506.
Bricout V, Germain P, Serrurier B, et al. Changes in testosterone muscle receptors: effects of an androgen treatment on physically trained rats. Cell Mol Biol. 1994;40(3):291–4.
Lu Y, Tong Q, He L. The effect of exercise on the androgen receptor binding capacity and the level of testosterone in the skeletal muscle. Chin J Appl Physiol. 1997;13(3):198–201.
Bamman MM, Shipp JR, Jiang J, et al. Mechanical load increases muscle IGF-I and androgen receptor mRNA concentrations in humans. Am J Physiol Endocrinol Metab. 2001;280(3):E383–90.
Deschenes MR, Maresh CM, Armstrong LE, et al. Endurance and resistance exercise induce muscle fiber type specific responses in androgen binding capacity. J Steroid Biochem Mol Biol. 1994;50(3):175–9.
Kadi F, Bonnerud P, Eriksson A, et al. The expression of androgen receptors in human neck and limb muscles: effects of training and self-administration of androgenic-anabolic steroids. Histochem Cell Biol. 2000;113(1):25–9.
Ratamess NA, Kraemer WJ, Volek JS, et al. Androgen receptor content following heavy resistance exercise in men. J Steroid Biochem Mol Biol. 2005;93(1):35–42.
Vingren JL, Kraemer WJ, Hatfield DL, et al. Effect of resistance exercise on muscle steroid receptor protein content in strength-trained men and women. Steroids. 2009;74(13):1033–9.
Vingren JL, Kraemer WJ, Ratamess NA, et al. Testosterone physiology in resistance exercise and training. Sports Med. 2010;40(12):1037–53.
Inoue K, Yamasaki S, Fushiki T, et al. Androgen receptor antagonist suppresses exercise-induced hypertrophy of skeletal muscle. Eur J Appl Physiol Occup Physiol. 1994;69(1):88–91.
Kvorning T, Andersen M, Brixen K, et al. Suppression of testosterone does not blunt mRNA expression of myoD, myogenin, IGF, myostatin or androgen receptor post strength training in humans. J Physiol. 2007;578(2):579–93.
Spangenburg EE, Le Roith D, Ward CW, et al. A functional insulin-like growth factor receptor is not necessary for load-induced skeletal muscle hypertrophy. J Physiol. 2008;586(1):283–91.
Rønnestad BR, Nygaard H, Raastad T. Physiological elevation of endogenous hormones results in superior strength training adaptation. Eur J Appl Physiol. 2011;111(9):2249–59.
West DW, Cotie LM, Mitchell CJ, et al. Resistance exercise order does not determine postexercise delivery of testosterone, growth hormone, and IGF-1 to skeletal muscle. Appl Physiol Nutr Metab. 2012;38(2):220–6.
Apró W, Blomstrand E. Influence of supplementation with branched-chain amino acids in combination with resistance exercise on p70s6 kinase phosphorylation in resting and exercising human skeletal muscle. Acta Physiol. 2010;200(3):237–48.
Deldicque L, De Bock K, Maris M, et al. Increased p70s6k phosphorylation during intake of a protein–carbohydrate drink following resistance exercise in the fasted state. Eur J Appl Physiol. 2010;108(4):791–800.
Farnfield MM, Carey KA, Gran P, et al. Whey protein ingestion activates mTOR-dependent signalling after resistance exercise in young men: a double-blinded randomized controlled trial. Nutrients. 2009;1(2):263–75.
Hulmi JJ, Tannerstedt J, Selänne H, et al. Resistance exercise with whey protein ingestion affects mTOR signaling pathway and myostatin in men. J Appl Physiol. 2009;106(5):1720–9.
Karlsson HK, Nilsson P-A, Nilsson J, et al. Branched-chain amino acids increase p70s6k phosphorylation in human skeletal muscle after resistance exercise. Am J Physiol Endocrinol Metab. 2004;287(1):E1–7.
Dreyer HC, Fujita S, Cadenas JG, et al. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol. 2006;576(2):613–24.
Dreyer HC, Fujita S, Glynn EL, et al. Resistance exercise increases leg muscle protein synthesis and mTOR signalling independent of sex. Acta Physiol. 2010;199(1):71–81.
Roschel H, Ugrinowistch C, Barroso R, et al. Effect of eccentric exercise velocity on Akt/mTOR/p70s6k signaling in human skeletal muscle. Appl Physiol Nutr Metab. 2011;36(2):283–90.
Areta JL, Burke LM, Ross ML, et al. Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. J Physiol. 2013;591(9):2319–31.
Glover EI, Oates BR, Tang JE, et al. Resistance exercise decreases eIF2B phosphorylation and potentiates the feeding-induced stimulation of p70s6k1 and rpS6 in young men. Am J Phys Reg Integr Compar Physiol. 2008;295(2):R604–10.
Terzis G, Spengos K, Mascher H, et al. The degree of p70s6k and s6 phosphorylation in human skeletal muscle in response to resistance exercise depends on the training volume. Eur J Appl Physiol. 2010;110(4):835–43.
Hulmi J, Walker S, Ahtiainen J, et al. Molecular signaling in muscle is affected by the specificity of resistance exercise protocol. Scand J Med Sci Sports. 2012;22(2):240–8.
Oishi Y, Tsukamoto H, Yokokawa T, et al. Mixed lactate and caffeine compound increases satellite cell activity and anabolic signals for muscle hypertrophy. J Appl Physiol. 2015;118(6):742–9.
Gundermann DM, Dickinson JM, Fry CS, et al. Inhibition of glycolysis and mTORC1 activation in human skeletal muscle with blood flow restriction exercise. FASEB J. 1076;2012(26):3.
Moore DR, Phillips SM, Babraj JA, et al. Myofibrillar and collagen protein synthesis in human skeletal muscle in young men after maximal shortening and lengthening contractions. Am J Physiol Endocrinol Metab. 2005;288(6):E1153–9.
Cuthbertson DJ, Babraj J, Smith K, et al. Anabolic signaling and protein synthesis in human skeletal muscle after dynamic shortening or lengthening exercise. Am J Physiol Endocrinol Metab. 2006;290(4):E731–8.
Eliasson J, Elfegoun T, Nilsson J, et al. Maximal lengthening contractions increase p70 s6 kinase phosphorylation in human skeletal muscle in the absence of nutritional supply. Am J Physiol Endocrinol Metab. 2006;291(6):E1197–205.
Rahbek SK, Farup J, Møller AB, et al. Effects of divergent resistance exercise contraction mode and dietary supplementation type on anabolic signalling, muscle protein synthesis and muscle hypertrophy. Amino Acids. 2014;46(10):2377–92.
Moore DR, Young M, Phillips SM. Similar increases in muscle size and strength in young men after training with maximal shortening or lengthening contractions when matched for total work. Eur J Appl Physiol. 2012;112(4):1587–92.
Davidsen PK, Gallagher IJ, Hartman JW, et al. High responders to resistance exercise training demonstrate differential regulation of skeletal muscle mRNA expression. J Appl Physiol. 2011;110(2):309–17.
Phillips SM. A brief review of critical processes in exercise-induced muscular hypertrophy. Sports Med. 2014;44(1):71–7.
Ogasawara R, Kobayashi K, Tsutaki A, et al. mTOR signaling response to resistance exercise is altered by chronic resistance training and detraining in skeletal muscle. J Appl Physiol. 2013;114(7):934–40.
Hoffman J, Maresh C, Armstrong L, et al. Effects of off-season and in-season resistance training programs on a collegiate male basketball team. J Hum Muscle Perform. 1991;1(2):48–55.
Häkkinen K, Komi PV, Alén M, et al. EMG, muscle fibre and force production characteristics during a 1 year training period in elite weight-lifters. Eur J Appl Physiol Occup Physiol. 1987;56(4):419–27.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this article.
Conflict of interest
Adam Gonzalez, Jay Hoffman, Jeffrey Stout, David Fukuda, and Darryn Willoughby declare that they have no conflicts of interest relevant to the content of this review.
Rights and permissions
About this article
Cite this article
Gonzalez, A.M., Hoffman, J.R., Stout, J.R. et al. Intramuscular Anabolic Signaling and Endocrine Response Following Resistance Exercise: Implications for Muscle Hypertrophy. Sports Med 46, 671–685 (2016). https://doi.org/10.1007/s40279-015-0450-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-015-0450-4