Abstract
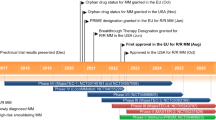
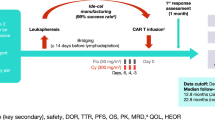
Elranatamab (elranatamab-bcmm; ELREXFIO™) is a bispecific B-cell maturation antigen (BCMA)-directed CD3 T cell engager being developed by Pfizer for the treatment of multiple myeloma (MM). Elranatamab bridges CD3 on T cells with BCMA expressed on multiple myeloma cells, thereby activating T cells to induce T cell-mediated cytotoxicity against myeloma cells. In August 2023, elranatamab received its first approval in the USA for the treatment of adult patients with relapsed or refractory multiple myeloma (RRMM) who have received at least four prior lines of therapy including a proteasome inhibitor, an immunomodulatory agent and an anti-CD38 monoclonal antibody. Elranatamab received accelerated approval for this indication based on response rate and durability of response, and continued approval may be contingent on the demonstration of clinical benefit in a confirmatory trial(s). Elranatamab has also received a positive opinion in the EU for RRMM and is under regulatory review in Japan and several other countries worldwide. Clinical studies of elranatamab are also underway in countries around the world. This article summarizes the milestones in the development of elranatamab leading to this first approval for the treatment of RRMM.
Similar content being viewed by others
References
van de Donk NWCJ, Zweegman S. T-cell-engaging bispecific antibodies in cancer. Lancet. 2023;402(10396):142–58.
Wang Q, Chen Y, Park J, et al. Design and production of bispecific antibodies. Antibodies (Basel). 2019;8(3):43.
Wei J, Yang Y, Wang G, Liu M. Current landscape and future directions of bispecific antibodies in cancer immunotherapy. Front Immunol. 2022;13:1035276.
US FDA. BLA accelerated approval (elranatamab-bcmm). 2023. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2023/761345Orig1s000ltr.pdf. Accessed 28 Aug 2023.
Pfizer Inc. ELREXFIOTM (elranatamab-bcmm): US prescribing information. 2023. https://labeling.pfizer.com/ShowLabeling.aspx?id=19669. Accessed 28 Aug 2023.
European Medicines Agency. CHMP summary of positive opinion for Elrexfio (elranatamab). 2023. https://www.ema.europa.eu/en/documents/smop-initial/chmp-summary-positive-opinion-elrexfio_en.pdf. Accessed 18 Oct 2023.
Bahlis NJ, Costello CL, Raje NS, et al. Elranatamab in relapsed or refractory multiple myeloma: the MagnetisMM-1 phase 1 trial. Nat Med. 2023. https://doi.org/10.1038/s41591-023-02589-w.
Dalovisio A, Bahlis N, Raje N, et al. Updated results from the ongoing phase 1 study of elranatamab, a BCMA targered T-cell redirecting immunotherapy, for patients with relapsed or refractory multiple myeloma [abstract no. P897]. HemaSphere. 2022;6(S3):1509–10.
Raje N, Bahlis NJ, Costello C, et al. Elranatamab, a BCMA targeted T-cell engaging bspecific antibody, induces durable clinical and molecular responses for patients with relapsed or refractory multiple myeloma [abstract]. Blood. 2022;140(Suppl 1):388–90.
Rodriguez-Otero P, Quach H, Weisel K, et al. Genomic analysis to identify determinants of inherent response and resistance to elranatamab in MagnetisMM-3 cohort A [abstract no. P906]. HemaSphere. 2023;7(S3):1709–10.
Elmeliegy M, Viqueira A, Vandendries E, et al. Dose optimization to mitigate the risk of CRS with elranatamab in multiple myeloma [abstract]. Blood. 2022;140(Supplement 1):7174–5.
Lesokhin AM, Tomasson MH, Arnulf B, et al. Elranatamab in relapsed or refractory multiple myeloma: phase 2 MagnetisMM-3 trial results. Nat Med. 2023. https://doi.org/10.1038/s41591-023-02528-9.
Manier S, Lesokhin A, Mohty M, et al. Efficacy and safety of elranatamab in patients with relapsed/refractory multiple myeloma and prior B-cell maturation antigen (BCMA)-directed therapies: a pooled analysis from MagnetisMM studies [abstract no. P870 plus poster]. HemaSphere. 2023;7(S3):1633–34.
Leleu X, Iida S, Landgren CO, et al. Elranatamab monotherapy or in combination with daratumumab vs daratumumab + pomalidomide + dexamethasone for patients with relapsed/refractory multiple myeloma: phase 3 MagnetisMM-5 Study, Part 2 [abstract no. P930]. HemaSphere. 2023;7(S3):1758.
Manteca MVM, Grosicki S, Kim K. Magnetismm-7: an open-label, multicenter, randomized phase 3 study of elranatamab versus lenalidomide in post-transplant patients with newly dagnosed multiple myeloma [abstract no. PB2131]. HemaSphere. 2023;7(S3):4084.
Manteca MVM, Grosicki S, Kim K. MagnetisMM-7: an open-label, multicenter, randomized phase 3 study of elranatamab versus lenalidomide in post-transplant patients with newly diagnosed multiple myeloma [abstract no. TPS8066]. J Clin Oncol. 2023;41(16 Suppl).
Landgren O, Kazandjian D, O’Connell A, et al. Magnetismm-4: an open label, phase 1b/2 umbrella study of elranatamab in combination with other anti-cancer treatments for patients with multiple myeloma. Blood. 2022;140(Suppl 1):10172–3.
Fonseca R, Kuroda J, Ishida T, et al. MagnetisMM-9: an open-label, multicenter, non-randomized phase 1/2 study of elranatamab in patients with relapsed/refractory multiple myeloma [abstract no. TPS8068]. J Clin Oncol. 2022;40(16 Suppl).
Fonseca R, Kuroda J, Ishida T, et al. MafnetisMM-9: an open-label, multicenter, non-randomized phase 1/2 study of elranatamab in patients with relapsed/refractory multiple myeloma [abstract no. PB2032]. HemaSphere. 2022;6(S3):3518.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Sohita Dhillon is a contracted employee of Adis International Ltd/Springer Nature and declares no relevant conflicts of interest. All authors contributed to this article and are responsible for its content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dhillon, S. Elranatamab: First Approval. Drugs 83, 1621–1627 (2023). https://doi.org/10.1007/s40265-023-01954-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-023-01954-w