Abstract
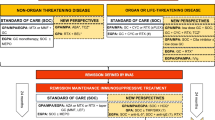
Avacopan (TAVNEOS™) is a complement 5a receptor (C5aR) antagonist developed by ChemoCentryx for the treatment of autoimmune diseases including anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis. The therapeutic effects of avacopan are attributed to the inhibition of C5aR activity on neutrophils, however, the exact mechanism of therapeutic efficacy in patients with ANCA-associated vasculitis has not been established. In September 2021, avacopan received its first approval in Japan for the treatment of microscopic polyangiitis (MPA) and granulomatosis with polyangiitis (GPA), the two most common forms of ANCA-associated vasculitis, where it is being commercialized by Kissei Pharmaceutical through a partnership with Vifor Pharma. In October 2021, avacopan was approved in the USA as an adjunctive treatment in adults for severe active ANCA-associated vasculitis (specifically MPA and GPA) in combination with standard therapy including glucocorticoids (avacopan does not eliminate glucocorticoid use). Avacopan has received a positive opinion in the EU, and is also undergoing regulatory review in Switzerland and Canada. Avacopan is being investigated for the treatment of complement component 3 glomerulopathy, hidradenitis suppurativa, lupus nephritis and IgA nephropathy. This article summarizes the milestones in the development of avacopan leading to these first approvals in Japan and the USA.
Similar content being viewed by others
References
ChemoCentryx. ChemoCentryx announces FDA approval of TAVNEOS™ (avacopan) in ANCA-associated vasculitis [media release]. 8 Oct 2021. https://ir.chemocentryx.com/.
Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013;65(1):1–11.
Jayne DRW, Merkel PA, Schall TJ, et al. Avacopan for the treatment of ANCA-associated vasculitis. N Engl J Med. 2021;384(7):599–609.
Kissei Pharmaceutical. Marketing Authorization Approval in Japan for TAVNEOS(Rm) capsules 10mg, a selective complement C5a receptor antagonist [media release]. 27 Sept 2021. http://www.kissei.co.jp.
ChemoCentryx. Avacopan (TAVNEOS): Japanese prescribing information. 2021. https://www.pmda.go.jp/. Accessed 05 Nov 2021.
ChemoCentryx. Avacopan (TAVNEOS): US prescribing information. 2021. https://www.chemocentryx.com/. Accessed 05 Nov 2021.
European Medicines Agency. First-in-class medicine recommended for treatment of rare blood vessel inflammation [media release]. 12 Nov 2021. https://www.ema.europa.eu/..
Vifor Pharma. Vifor Pharma reports strong H1 2021 growth, on track to meet full year guidance [media release]. 5 Aug 2021. http://viforpharma.com.
GlaxoSmithKline, ChemoCentryx Inc. GlaxoSmithKline and ChemoCentryx enter into drug discovery and development alliance in inflammatory disorders [media release]. 24 Aug 2006. http://www.gsk.com.
ChemoCentryx. ChemoCentryx announces positive top-line CCX168 phase II data in ANCA-associated renal vasculitis [media release]. 3 Dec 2013. https://ir.chemocentryx.com/.
Galenica Group. Galenica 2016: further increase in sales and confirmation of profit forecasts [media release]. 19 Jan 2017. http://www.galenica.com.
Vifor Pharma. Vifor Pharma licenses rights to commercialize ChemoCentryxs orally-administered complement 5aR inhibitor CCX168 for orphan and rare renal diseases in Europe and certain other major markets [media release]. 10 May 2016. http://www.viforpharma.com.
Vifor Pharma. Vifor Pharma and ChemoCentryx announce expansion of avacopan agreement for rare renal diseases [media release]. 14 Feb 2017. http://viforpharma.com.
ChemoCentryx. ChemoCentryx reports second quarter 2018 financial results and recent highlights [media release]. 9 Aug 2018. https://ir.chemocentryx.com/.
Vifor Fresenius Medical Care Renal Pharma. Kissei to market avacopan in Japan for Vifor Fresenius Medical Care Renal Pharma [media release]. 9 Jun 2017. http://viforpharma.com.
ChemoCentryx. Form 10-K annual report. 2021. https://ir.chemocentryx.com/. Accessed 05 Nov 2021.
Bekker P, Dairaghi D, Seitz L, et al. Characterization of pharmacologic and pharmacokinetic properties of CCX168, a potent and selective orally administered complement 5a receptor inhibitor, based on preclinical evaluation and randomized phase 1 clinical study. PLoS One. 2016;11(10):e0164646.
Xiao H, Dairaghi DJ, Powers JP, et al. C5a receptor (CD88) blockade protects against MPO-ANCA GN. J Am Soc Nephrol. 2014;25(2):225–31.
Gastoldi S, Charo IF, Dairaghi D, et al. Inhibition of the C5A receptor by CCX168 markedly reduces the thrombogenic potential of serum from patients with atypical hemolytic uremic syndrome [abstract no. SP004]. Nephrol Dial Transplant. 2015;30(Suppl 3):iii381–92.
US National Institutes of Health. ClinicalTrials.gov record for NCT02464891. 2017. https://clinicaltrials.gov/. Accessed 05 Nov 2021.
Jayne DRW, Merkel PA, Bekker P. Avacopan for the treatment of ANCA-associated vasculitis. Reply. N Engl J Med. 2021;384(21):e81.
Merkel PA, Niles J, Jimenez R, et al. Adjunctive treatment with avacopan, an oral C5a receptor inhibitor, in patients with antineutrophil cytoplasmic antibody-associated vasculitis. ACR Open Rheumatol. 2020;2(11):662–71.
Jayne DRW, Bruchfeld AN, Harper L, et al. Randomized trial of C5a receptor inhibitor avacopan in ANCA-associated vasculitis. J Am Soc Nephrol. 2017;28(9):2756–67.
Vifor Fresenius Medical Care Renal Pharma, ChemoCentryx Inc. VFMCRP and ChemoCentryx provide topline results from ACCOLADE trial of avacopan in C3 glomerulopathy including improved estimated glomerular filtration rate (eGFR) [media release]. 21 Dec 2020. http://viforpharma.com.
ChemoCentryx. ChemoCentryx announces positive topline results of phase II AURORA clinical trial of avacopan in the treatment of hidradenitis suppurativa (HS) [media release]. 28 Oct 2020. https://ir.chemocentryx.com/.
Bruchfeld A, Nachman PH, Parikh S, et al. C5A receptor inhibitor avacopan in IgA nephropathy—a pilot study [abstract]. Kidney Dis. 2018;4(3):140–1.
ChemoCentryx. Data on file. 2021
ChemoCentryx. ChemoCentryx reports first quarter 2021 financial results and recent highlights [media release]. 29 Apr 2021. https://ir.chemocentryx.com/.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. A. Lee is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, A. Avacopan: First Approval. Drugs 82, 79–85 (2022). https://doi.org/10.1007/s40265-021-01643-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-021-01643-6