Abstract
Background
In treating allergic rhinitis, montelukast has the potential to be used as an alternative or addition to an oral antihistamine or intranasal corticosteroid.
Objective
The objective of this systematic review was to assess the effectiveness of montelukast in treating allergic rhinitis.
Methods
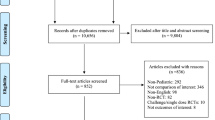
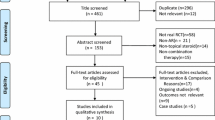
An electronic literature search was performed using the Cochrane Central Register of Controlled Trials, EMBASE, and MEDLINE from 1966 to 21 January 2019. The eligibility criteria were randomized controlled trials comparing montelukast with placebo or other standard treatments. The primary outcomes assessed were daytime nasal symptom score (DNS) and night-time nasal symptom score (NNS). The secondary outcomes assessed were composite nasal symptom score (CSS), daytime eyes symptom score (DES), and rhinoconjunctivitis quality-of-life questionnaires (RQLQ). The meta-analysis was conducted using Review Manager 5.3 software based on the random-effects model.
Results
Fifteen studies of 10387 participants met the inclusion criteria. Montelukast was more effective than placebo in improving DNS (mean difference [MD] − 0.12, 95% confidence interval [CI] − 0.15 to − 0.08; p < 0.001), NNS (MD − 0.09, 95% CI − 0.13 to − 0.05; p < 0.001), CSS (MD − 0.08, 95% CI − 0.11 to − 0.06; p < 0.001), DES (MD − 0.17, 95% CI − 0.33 to − 0.02; p < 0.030), and RQLQ (MD − 0.34, 95% CI − 0.49 to − 0.20; p < 0.001). Oral antihistamine was superior to montelukast in improving DNS (MD 0.08, 95% CI 0.03–0.13; p = 0.002), CSS (MD 0.03, 95% CI − 0.02 to 0.07; p = 0.27), DES (MD 0.06, 95% CI 0–0.12; p = 0.040), and RQLQ (MD 0.03, 95% CI − 0.05 to 0.12; p = 0.430). Montelukast was superior to oral antihistamine in improving NNS (MD -0.03, 95% CI − 0.08 to 0.03; p = 0.330). Intranasal fluticasone spray was superior to montelukast in improving DNS (MD 0.71, 95% CI 0.44–0.99; p < 0.001) and NNS (MD 0.63, 95% CI 0.29–0.97; p < 0.001). Combined montelukast and oral antihistamine was superior to oral antihistamine in improving DNS (MD − 0.15, 95% CI − 0.27 to − 0.03; p = 0.010), NNS (MD − 0.16, 95% CI − 0.28 to − 0.05; p = 0.006), CSS (MD − 0.12, 95% CI − 0.25 to − 0.01; p = 0.070), DES (MD − 0.12, 95% CI − 0.30 to 0.06; p = 0.180), and RQLQ (MD − 0.10, 95% CI − 0.28 to 0.08; p = 0.290). Combined montelukast and OAH was superior to montelukast in improving DNS (MD 0.15, 95% CI 0.08–0.21; p < 0.001), NNS (MD 0.05, 95% CI − 0.09 to 0.19; p = 0.510), CSS (MD 0.1, 95% CI 0.03–0.17; p = 0.007), DES (MD 0.18, 95% CI 0–0.36; p = 0.050), and RQLQ (MD 0.07 95% CI − 0.15 to 0.29; p = 0.530).
Conclusions
Montelukast is more effective than placebo in treating the overall symptoms of allergic rhinitis while the combined therapy of montelukast and an oral antihistamine is superior to either montelukast or an oral antihistamine alone.
























Similar content being viewed by others
References
Braido F, Riccio AM, Rogkakou A, Massacane P, Guerra L, Fumagalli F, et al. Montelukast effects on inflammation in allergic rhinitis: a double blind placebo controlled pilot study. Eur Ann Allergy Clin Immunol. 2012;44(2):48–53.
Santos CB, Hanks C, McCann J, Lehman EB, Pratt E, Craig TJ. The role of montelukast on perennial allergic rhinitis and associated sleep disturbances and daytime somnolence. Allergy Asthma Proc. 2008;29(2):140–5. https://doi.org/10.2500/aap.2008.29.3097.
Chen H, Lou H, Wang Y, Cao F, Zhang L, Wang C. Comparison of the efficacy and mechanisms of intranasal budesonide, montelukast, and their combination in treatment of patients with seasonal allergic rhinitis. Int Forum Allergy Rhinol. 2018;8(11):1242–52. https://doi.org/10.1002/alr.22197.
Dalgic A, Dinc ME, Ulusoy S, Dizdar D, Is A, Topak M. Comparison of the effects of nasal steroids and montelukast on olfactory functions in patients with allergic rhinitis. Eur Ann Otorhinolaryngol Head Neck Dis. 2017;134(4):213–6. https://doi.org/10.1016/j.anorl.2016.05.012.
Okubo K, Kurono Y, Fujieda S, Ogino S, Uchio E, Odajima H, et al. Japanese guideline for allergic rhinitis 2014. Allergol Int. 2014;63(3):357–75. https://doi.org/10.2332/allergolint.14-rai-0768.
Santanello NC, DeMuro-Mercon C, Shah SR, Schenkel EJ, Ratner PH, Dass SB, et al. Validation of the nighttime symptoms score as a clinically relevant measure of allergic rhinitis. Allergy Asthma Proc. 2006;27(3):231–9.
Ciebiada M, Gorska-Ciebiada M, Barylski M, Kmiecik T, Gorski P. Use of montelukast alone or in combination with desloratadine or levocetirizine in patients with persistent allergic rhinitis. Am J Rhinol Allergy. 2011;25(1):e1–6. https://doi.org/10.2500/ajra.2011.25.3540.
Wei C. The efficacy and safety of H1-antihistamine versus montelukast for allergic rhinitis: a systematic review and meta-analysis. Biomed Pharmacother. 2016;83:989–97. https://doi.org/10.1016/j.biopha.2016.08.003.
Xiao J, Wu WX, Ye YY, Lin WJ, Wang L. A network meta-analysis of randomized controlled trials focusing on different allergic rhinitis medications. Am J Ther. 2016;23(6):e1568–78. https://doi.org/10.1097/mjt.0000000000000242.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. 2nd ed. Chichester: Wiley; 2019.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9.
Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ, et al. What is “quality of evidence” and why is it important to clinicians? BMJ. 2008;336(7651):995–8. https://doi.org/10.1136/bmj.39490.551019.be.
Goh BS, Ismail MI, Husain S. Quality of life assessment in patients with moderate to severe allergic rhinitis treated with montelukast and/or intranasal steroids: a randomized, double-blind, placebo-controlled study. J Laryngol Otol. 2014;128(3):242–8. https://doi.org/10.1017/s002221511400036x.
Gupta V, Matreja PS. Efficacy of montelukast and levocetirizine as treatment for allergic rhinitis. J Allergy Ther. 2010;1:103. https://doi.org/10.4172/2155-6121.1000103.
van Adelsberg J, Philip G, Pedinoff AJ, Meltzer EO, Ratner PH, Menten J, et al. Montelukast improves symptoms of seasonal allergic rhinitis over a 4-week treatment period. Allergy. 2003;58(12):1268–76. https://doi.org/10.1046/j.1398-9995.2003.00261.x.
van Adelsberg J, Philip G, LaForce CF, Weinstein SF, Menten J, Malice MP, et al. Randomized controlled trial evaluating the clinical benefit of montelukast for treating spring seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2003;90(2):214–22. https://doi.org/10.1016/s1081-1206(10)62144-8.
Patel P, Philip G, Yang W, Call R, Horak F, LaForce C, et al. Randomized, double-blind, placebo-controlled study of montelukast for treating perennial allergic rhinitis. Ann Allergy Asthma Immunol. 2005;95(6):551–7. https://doi.org/10.1016/s1081-1206(10)61018-6.
Pullerits T, Praks L, Ristioja V, Lötvall J. Comparison of a nasal glucocorticoid, antileukotriene, and a combination of antileukotriene and antihistamine in the treatment of seasonal allergic rhinitis. J Allergy Clin Immunol. 2002;109(6):949–55. https://doi.org/10.1067/mai.2002.124467.
Meltzer EO, Malmstrom K, Lu S, Prenner BM, Wei LX, Weinstein SF, et al. Concomitant montelukast and loratadine as treatment for seasonal allergic rhinitis: a randomized, placebo-controlled clinical trial. J Allergy Clin Immunol. 2000;105(5):917–22. https://doi.org/10.1067/mai.2000.106040.
Lu S, Malice MP, Dass SB, Reiss TF. Clinical studies of combination montelukast and loratadine in patients with seasonal allergic rhinitis. J Asthma. 2009;46(9):878–83. https://doi.org/10.3109/02770900903104540.
Katial RK, Oppenheimer JJ, Ostrom NK, Mosnaim GS, Yancey SW, Waitkus-Edwards KR, et al. Adding montelukast to fluticasone propionate/salmeterol for control of asthma and seasonal allergic rhinitis. Allergy Asthma Proc. 2010;31(1):68–75. https://doi.org/10.2500/aap.2010.31.3306.
Martin BG, Andrews CP, van Bavel JH, Hampel FC, Klein KC, Prillaman BA, et al. Comparison of fluticasone propionate aqueous nasal spray and oral montelukast for the treatment of seasonal allergic rhinitis symptoms. Ann Allergy Asthma Immunol. 2006;96(6):851–7. https://doi.org/10.1016/s1081-1206(10)61349-x.
Nayak AS, Philip G, Lu S, Malice MP, Reiss TF. Efficacy and tolerability of montelukast alone or in combination with loratadine in seasonal allergic rhinitis: a multicenter, randomized, double-blind, placebo-controlled trial performed in the fall. Ann Allergy Asthma Immunol. 2002;88(6):592–600. https://doi.org/10.1016/s1081-1206(10)61891-1.
Cingi C, Ozlugedik S. Effects of montelukast on quality of life in patients with persistent allergic rhinitis. Otolaryngol Head Neck Surg. 2010;142(5):654–8. https://doi.org/10.1016/j.otohns.2010.01.016.
Philip G, Malmstrom K, Hampel FC, Weinstein SF, LaForce CF, Ratner PH, et al. Montelukast for treating seasonal allergic rhinitis: a randomized, double-blind, placebo-controlled trial performed in the spring. Clin Exp Allergy. 2002;32(7):1020–8.
Philip G, Nayak AS, Berger WE, Leynadier F, Vrijens F, Dass SB, et al. The effect of montelukast on rhinitis symptoms in patients with asthma and seasonal allergic rhinitis. Curr Med Res Opin. 2004;20(10):1549–58. https://doi.org/10.1185/030079904x3348.
Juniper EF, Guyatt GH. Development and testing of a new measure of health status for clinical trials in rhinoconjunctivitis. Clin Exp Allergy. 1991;21(1):77–83. https://doi.org/10.1111/j.1365-2222.1991.tb00807.x.
Abdullah B, Mutalib NS, Mohamad H. Night time symptoms and day time sleepiness among allergic rhinitis patients. Pan Arab J Rhinol. 2017;7:1–6.
Storms WW. Pharmacologic approaches to daytime and nighttime symptoms of allergic rhinitis. J Allergy Clin Immunol. 2004;114(5S):S146–53. https://doi.org/10.1016/j.jaci.2004.08.045.
Kim H, Bouchard J, Renzi PM. The link between allergic rhinitis and asthma: a role for antileukotrienes? Can Respir J. 2008;15(2):91–8. https://doi.org/10.1155/2008/416095.
Tan NC, Nadkarni NV, Lye WK, Sankari U, Nguyen VH. Ten-year longitudinal study of factors influencing nocturnal asthma symptoms among Asian patients in primary care. NPJ Prim Care Respir Med. 2015;25:15064. https://doi.org/10.1038/npjpcrm.2015.64.
Brożek JL, Bousquet J, Agache I, Agarwal A, Bachert C, Bosnic-Anticevich S, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2016 revision. J Allergy Clin Immunol. 2017;140(4):950–8. https://doi.org/10.1016/j.jaci.2017.03.050.
Baharudin A, Abdul Latiff AH, Woo K, Yap FB, Tang IP, Leong KF, et al. Using patient profiles to guide the choice of antihistamines in the primary care setting in Malaysia: expert consensus and recommendations. Ther Clin Risk Manag. 2019;15:1267–75. https://doi.org/10.2147/tcrm.s221059.
Wallace DV, Dykewicz MS, Bernstein DI, Blessing-Moore J, Cox L, Khan DA, et al. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008;122(2 Suppl.):S1–84. https://doi.org/10.1016/j.jaci.2008.06.003.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the preparation of this article.
Conflict of Interest
Madhusudhan Krishnamoorthy, Norhayati Mohd Noor, Norhafiza Mat Lazim, and Baharudin Abdullah have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
MK: conception, development of the protocol, study selection, data extraction, assessment of the risk of bias, data analysis and interpretation, manuscript preparation, critical review, final approval. NMN: conception, development of the protocol, study selection, data extraction, assessment of the risk of bias, data analysis and interpretation, manuscript preparation, critical review, final approval. NML: conception, development of the protocol, study selection, data extraction, assessment of the risk of bias, data analysis and interpretation, manuscript preparation, critical review, final approval. BA: conception, development of the protocol, study selection, data extraction, assessment of risk of the bias, data analysis and interpretation, manuscript preparation, critical review, final approval.
Rights and permissions
About this article
Cite this article
Krishnamoorthy, M., Mohd Noor, N., Mat Lazim, N. et al. Efficacy of Montelukast in Allergic Rhinitis Treatment: A Systematic Review and Meta-Analysis. Drugs 80, 1831–1851 (2020). https://doi.org/10.1007/s40265-020-01406-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-020-01406-9