Abstract
Dupilumab (Dupixent®) is a fully human monoclonal antibody directed against the interleukin (IL)-4 receptor α (IL-4Rα) subunit. Dupilumab inhibits the signalling of the type 2 cytokines IL-4 and IL-13 and was co-developed by Regeneron Pharmaceuticals and Sanofi as a potential therapeutic agent for the treatment of atopic or allergic diseases. In March 2017 dupilumab received its first global approval, in the USA, for use in the treatment of adult patients with moderate-to-severe atopic dermatitis whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. Dupilumab is in preregistration for this indication in the EU. In addition, dupilumab is currently under phase III development across the world for the treatment of asthma and nasal polyposis as well as for atopic dermatitis in paediatric patients. The agent has also entered phase II development in the USA for the treatment of eosinophilic oesophagitis. This article summarizes the milestones in the development of dupilumab leading to this first approval for the treatment of moderate-to-severe atopic dermatitis in adults.
Similar content being viewed by others
References
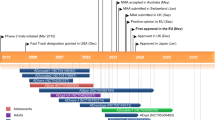
US FDA. Dupixent® (dupilumab) injection, for subcutaneous use: US prescribing information. 2017. https://www.fda.gov. Accessed 02 May 2017.
Sanofi. Sanofi and Regeneron announce FDA approval of Dupixent® (dupilumab), the first targeted biologic therapy for adults with moderate-to-severe atopic dermatitis (media release). 2017. http://mediaroom.sanofi.com/press-releases/. Accessed 02 May 2017.
Sanofi. Sanofi and Regeneron announce Marketing Authorization Application for Dupixent® (dupilumab) accepted for review by the EMA (media release). 2016. http://mediaroom.sanofi.com/press-releases/. Accessed 02 May 2017.
Sanofi. UK patients granted early access to Sanofi’s innovative dermatology treatment dupilumab by MHRA (media release). 2017. http://www.sanofi.co.uk. Accessed 02 May 2017.
Regeneron Pharmaceuticals. Regeneron reports third quarter 2016 financial and operating results (media release). 2016. http://newsroom.regeneron.com/releases.cfm?view=all. Accessed 02 May 2017.
Beck LA, Thaçi D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371(2):130–9.
Swanson BN, Mannent L, Hamilton JD, et al. The effect of dupilumab on biomarkers in the peripheral blood and nasal secretions in the treatment of chronic sinusitis with nasal polyposis (abstract no. B041). Inflamm Res. 2015;64(2 Suppl):S118–9.
Wenzel S, Castro M, Corren J, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. 2016;388(10039):31–44.
Wenzel S, Ford L, Pearlman D, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368(26):2455–66.
Bachert C, Mannent L, Naclerio RM, et al. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA. 2016;315(5):469–79.
Blauvelt A, Simpson E, Wu R, et al. The effect of dupilumab on vaccine antibody responses in adults with moderate-to-severe atopic dermatitis: a randomized, double-blind, placebo-controlled trial (abstract no. 1347). Allergy. 2016;71(Suppl 102):95.
Kovalenko P, DiCioccio AT, Davis JD, et al. Exploratory population PK analysis of dupilumab, a fully human monoclonal antibody against IL-4Rα, in atopic dermatitis patients and normal volunteers. CPT Pharmacomet Syst Pharmacol. 2016;5(11):617–24.
Davis JD, Rawal S, Kamal M, et al. Pharmacokinetics of dupilumab in long-term phase III studies in adult patients with moderate-to-severe atopic dermatitis (abstract no. PII-029). Clin Pharmacol Ther. 2017;101(Suppl 1):S61.
Cork MJ. Pharmacokinetics, safety and efficacy of dupilumab in a pediatric population with moderate-to-severe atopic dermatitis: results from an open-label phase 2a trial (abstract no. 5279). Annual Meeting of the American Academy of Dermatology. 2017.
Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335–48.
Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017. doi:10.1016/S0140-6736(17)31191-1.
Simpson EL, Gadkari A, Worm M, et al. Dupilumab therapy provides clinically meaningful improvement in patient-reported outcomes (PROs): a phase IIb, randomized, placebo-controlled, clinical trial in adult patients with moderate to severe atopic dermatitis (AD). J Am Acad Dermatol. 2016;75(3):506–15.
Thaçi D, Simpson EL, Beck LA, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2016;387(10013):40–52.
Deleuran M, Thaçi D, Beck LA, et al. Long-term safety and efficacy of open-label dupilumab in patients with moderate-to-severe atopic dermatitis (abstract no. L21). J Allergy Clin Immunol. 2017;139(2 Suppl):AB381.
US FDA. Dupixent (dupilumab): medical review. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/761055Orig1s000MedR.pdf. Accessed 19 May 2017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the author on the basis of scientific completeness and accuracy. M. Shirley is a salaried employee of Adis, Springer SBM. Additional information about this AdisInsight Report can be found http://www.medengine.com/Redeem/EE48F06062D9A22D.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Shirley, M. Dupilumab: First Global Approval. Drugs 77, 1115–1121 (2017). https://doi.org/10.1007/s40265-017-0768-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-017-0768-3