Abstract
Background and Objective
Ketamine may work as an anti-inflammatory agent, and it increases the levels of vascular endothelial growth factor (VEGF) in patients with treatment-resistant depression. However, whether genes related to pro-inflammatory and anti-inflammatory cytokines and VEGF may predict the treatment response to ketamine remains unknown.Therefore the aim of this study was to analyze whether specific genes related to inflammatory processes and VEGF were associated with treatment response to low-dose ketamine in patients with treatment-resistant depression.
Methods
Based on the genome data from our clinical trial, this study was a secondary analysis of candidate genes correlated with different timepoints of depressive symptoms. In total, 65 patients with treatment-resistant depression (n = 21 for ketamine 0.5 mg/kg, 20 for ketamine 0.2 mg/kg, and 24 for normal saline) were genotyped for 684,616 single nucleotide polymorphisms. Genes associated with 80 cytokines (i.e., interleukin [IL]-1, IL-6, tumor necrosis factor-α, and adiponectin) and VEGF (i.e., VEGF and VEGF receptors) were selected for the gene-based genome-wide association study on the antidepressant effect of a ketamine infusion.
Results
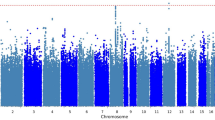
Specific single nucleotide polymorphisms, including rs2540315 and rs75746675 in IL1R1 and rs79568085 in VEGFC, were related to the rapid (within 240 min) antidepressant effect of a ketamine infusion; specific single nucleotide polymorphisms, such as Affx-20131665 in PIGF and rs8179353, rs8179353, and rs8179353 in TNFRSF8, were associated with the sustained (up to 2 weeks) antidepressant effect of low-dose (combined 0.5 mg/kg and 0.2 mg/kg) ketamine.
Conclusions
Our findings further revealed that genes related to both anti-inflammatory and pro-inflammatory cytokines (i.e., IL-1, IL-2, IL-6, tumor necrosis factor-α, C-reactive protein, and adiponectin) and VEGF-FLK signaling predicted the treatment response to a ketamine infusion in patients with treatment-resistant depression. The synergic modulation of inflammatory and VEGF systems may contribute to the antidepressant effect of ketamine.
Clinical Trial Registration
University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR) number: UMIN000016985.
Similar content being viewed by others
References
Chen MH, Li CT, Lin WC, Hong CJ, Tu PC, Bai YM, et al. Persistent antidepressant effect of low-dose ketamine and activation in the supplementary motor area and anterior cingulate cortex in treatment-resistant depression: a randomized control study. J Affect Disord. 2018;225:709–14.
Li CT, Chen MH, Lin WC, Hong CJ, Yang BH, Liu RS, et al. The effects of low-dose ketamine on the prefrontal cortex and amygdala in treatment-resistant depression: a randomized controlled study. Hum Brain Mapp. 2016;37:1080–90.
Su TP, Chen MH, Li CT, Lin WC, Hong CJ, Gueorguieva R, et al. Dose-related effects of adjunctive ketamine in Taiwanese patients with treatment-resistant depression. Neuropsychopharmacology. 2017;42:2482–92.
Marcantoni WS, Akoumba BS, Wassef M, Mayrand J, Lai H, Richard-Devantoy S, et al. A systematic review and meta-analysis of the efficacy of intravenous ketamine infusion for treatment resistant depression: January 2009 - January 2019. J Affect Disord. 2020;277:831–41.
Zhou Y, Wang C, Lan X, Zheng W, Li H, Chao Z, et al. The effectiveness of repeated intravenous ketamine on subjective and objective psychosocial function in patients with treatment-resistant depression and suicidal ideation. J Affect Disord. 2022;304:78–84.
Phillips JL, Norris S, Talbot J, Birmingham M, Hatchard T, Ortiz A, et al. Single, repeated, and maintenance ketamine infusions for treatment-resistant depression: a randomized controlled trial. Am J Psychiatry. 2019;176:401–9.
Nikayin S, Murphy E, Krystal JH, Wilkinson ST. Long-term safety of ketamine and esketamine in treatment of depression. Expert Opin Drug Saf. 2022;21(6):777–87.
Lengvenyte A, Strumila R, Olie E, Courtet P. Ketamine and esketamine for crisis management in patients with depression: why, whom, and how? Eur Neuropsychopharmacol. 2022;57:88–104.
Kiraly DD, Horn SR, Van Dam NT, Costi S, Schwartz J, Kim-Schulze S, et al. Altered peripheral immune profiles in treatment-resistant depression: response to ketamine and prediction of treatment outcome. Transl Psychiatry. 2017;7:e1065.
Nikkheslat N. Targeting inflammation in depression: Ketamine as an anti-inflammatory antidepressant in psychiatric emergency. Brain Behav Immun Health. 2021;18:100383.
De Kock M, Loix S, Lavand’homme P. Ketamine and peripheral inflammation. CNS Neurosci Ther. 2013;19:403–10.
Chen MH, Li CT, Lin WC, Hong CJ, Tu PC, Bai YM, et al. Rapid inflammation modulation and antidepressant efficacy of a low-dose ketamine infusion in treatment-resistant depression: a randomized, double-blind control study. Psychiatry Res. 2018;269:207–11.
Liu JJ, Wei YB, Strawbridge R, Bao Y, Chang S, Shi L, et al. Peripheral cytokine levels and response to antidepressant treatment in depression: a systematic review and meta-analysis. Mol Psychiatry. 2020;25:339–50.
Atake K, Hori H, Kageyama Y, Koshikawa Y, Igata R, Tominaga H, et al. Pre-treatment plasma cytokine levels as potential predictors of short-term remission of depression. World J Biol Psychiatry. 2022;23(10):785–93.
Park M, Newman LE, Gold PW, Luckenbaugh DA, Yuan P, Machado-Vieira R, et al. Change in cytokine levels is not associated with rapid antidepressant response to ketamine in treatment-resistant depression. J Psychiatr Res. 2017;84:113–8.
Zhou Y, Wang C, Lan X, Li H, Chao Z, Ning Y. Plasma inflammatory cytokines and treatment-resistant depression with comorbid pain: improvement by ketamine. J Neuroinflammation. 2021;18:200.
Machado-Vieira R, Gold PW, Luckenbaugh DA, Ballard ED, Richards EM, Henter ID, et al. The role of adipokines in the rapid antidepressant effects of ketamine. Mol Psychiatry. 2017;22:127–33.
Deyama S, Bang E, Kato T, Li XY, Duman RS. Neurotrophic and antidepressant actions of brain-derived neurotrophic factor require vascular endothelial growth factor. Biol Psychiatry. 2019;86:143–52.
Deyama S, Bang E, Wohleb ES, Li XY, Kato T, Gerhard DM, et al. Role of neuronal VEGF signaling in the prefrontal cortex in the rapid antidepressant effects of ketamine. Am J Psychiatry. 2019;176:388–400.
McGrory CL, Ryan KM, Gallagher B, McLoughlin DM. Vascular endothelial growth factor and pigment epithelial-derived factor in the peripheral response to ketamine. J Affect Disord. 2020;273:380–3.
Lazzeroni LC, Ray A. The cost of large numbers of hypothesis tests on power, effect size and sample size. Mol Psychiatry. 2012;17:108–14.
Guo W, Machado-Vieira R, Mathew S, Murrough JW, Charney DS, Grunebaum M, et al. Exploratory genome-wide association analysis of response to ketamine and a polygenic analysis of response to scopolamine in depression. Transl Psychiatry. 2018;8:280.
Mortezaei Z, Tavallaei M. Novel directions in data pre-processing and genome-wide association study (GWAS) methodologies to overcome ongoing challenges. Inform Med Unlocked. 2021;24:100586.
Wang DX, Kaur Y, Alyass A, Meyre D. A candidate-gene approach identifies novel associations between common variants in/near syndromic obesity genes and BMI in pediatric and adult European populations. Diabetes. 2019;68:724–32.
Chen MH, Kao CF, Tsai SJ, Li CT, Lin WC, Hong CJ, et al. Treatment response to low-dose ketamine infusion for treatment-resistant depression: a gene-based genome-wide association study. Genomics. 2021;113:507–14.
Broglio K. Randomization in clinical trials: permuted blocks and stratification. JAMA. 2018;319:2223–4.
Montgomery SA, Smeyatsky N, de Ruiter M, Montgomery DB. Profiles of antidepressant activity with the Montgomery-Asberg Depression Rating Scale. Acta Psychiatr Scand Suppl. 1985;320:38–42.
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62.
Liu JZ, McRae AF, Nyholt DR, Medland SE, Wray NR, Brown KM, et al. A versatile gene-based test for genome-wide association studies. Am J Hum Genet. 2010;87:139–45.
Wei Y, Chang L, Hashimoto K. Molecular mechanisms underlying the antidepressant actions of arketamine: beyond the NMDA receptor. Mol Psychiatry. 2022;27:559–73.
Yang JJ, Wang N, Yang C, Shi JY, Yu HY, Hashimoto K. Serum interleukin-6 is a predictive biomarker for ketamine’s antidepressant effect in treatment-resistant patients with major depression. Biol Psychiatry. 2015;77:e19-20.
Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, et al. Major depressive disorder. Nat Rev Dis Primers. 2016;2:16065.
Carmody TJ, Rush AJ, Bernstein I, Warden D, Brannan S, Burnham D, et al. The Montgomery Asberg and the Hamilton ratings of depression: a comparison of measures. Eur Neuropsychopharmacol. 2006;16:601–11.
Chen MH, Li CT, Lin WC, Hong CJ, Tu PC, Bai YM, et al. Cognitive function of patients with treatment-resistant depression after a single low dose of ketamine infusion. J Affect Disord. 2018;241:1–7.
Phillips JL, Van Geel A, Burhunduli P, Vasudev D, Batten LA, Norris S, et al. Assessment of objective and subjective cognitive function in patients with treatment-resistant depression undergoing repeated ketamine infusions. Int J Neuropsychopharmacol. 2022;25(12):992–1002.
Acknowledgements
We thank all research assistants, physicians, pharmacists, and nursing staff at D020 Unit of Taipei Veterans General Hospital for their assistance during the study process. We thank Mr I-Fan Hu, M.A. (Courtauld Institute of Art, University of London; National Taiwan University) for his friendship and support in English editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study was supported by grant from Taipei Veterans General Hospital (V111C-010, V111C-040, V111C-029), Yen Tjing Ling Medical Foundation (CI-109-21, CI-109-22, CI-110-30), Ministry of Science and Technology, Taiwan (MOST110-2314-B-075-026, MOST110-2314-B-075-024-MY3, MOST109-2314-B-010-050-MY3, MOST111-2314-B-075-014-MY2, MOST111-2314-B-075 -013), Taipei, Taichung, Kaohsiung Veterans General Hospital, Tri-Service General Hospital, Academia Sinica Joint Research Program (VTA112-V1-6-1) and Veterans General Hospitals and University System of Taiwan Joint Research Program (VGHUST112-G1-8-1). The funding source had no role in any process of our study.
Conflicts of Interest
Shih-Jen Tsai, Chung-Feng Kao, Tung-Ping Su, Cheng-Ta Li, Wei-Chen Lin, Chen-Jee Hong, Ya-Mei Bai, Pei-Chi Tu, and Mu-Hong Chen have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
This study was performed in accordance with the Declaration of Helsinki and was approved by the Taipei Veterans General Hospital Institutional Review Board. This clinical trial is registered at UMIN Clinical Trials Registry (UMIN-CTR) [trial registration number: UMIN000016985].
Consent to Participate
Informed consent was provided by all of the participants.
Consent for Publication
No identified confidential data were included in the study.
Availability of Data and Material
The datasets generated and/or analyzed during the current study are not publicly available because of Taiwan’s clinical trial ethical regulation but are available from the corresponding author on reasonable request.
Code Availability
Analytic codes are available from the corresponding author on reasonable request.
Authors’ Contributions
Conceptualization: MHC and TPS; methodology: MHC, TPS, SJT, and CFK; formal analysis: MHC and CFK; investigation: MHC, TPS, CTL, and WCL; writing, original draft: MHC, SJT, and CFK; writing, review and editing: all authors. All authors have read and approved the final submitted manuscript, and agree to be accountable for the work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tsai, SJ., Kao, CF., Su, TP. et al. Cytokine- and Vascular Endothelial Growth Factor-Related Gene-Based Genome-Wide Association Study of Low-Dose Ketamine Infusion in Patients with Treatment-Resistant Depression. CNS Drugs 37, 243–253 (2023). https://doi.org/10.1007/s40263-023-00989-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-023-00989-7