Abstract
Background and Objectives
Atezolizumab has demonstrated safety and efficacy in patients with metastatic non-small cell lung cancer (NSCLC) in the IMpower110 trial. The aim of this study was to evaluate the cost-effectiveness of atezolizumab as the first-line treatment for patients with unresectable advanced NSCLC, including programmed cell death ligand-1 (PD-L1)-positive probability testing, from the perspective of healthcare costs in Japan.
Methods
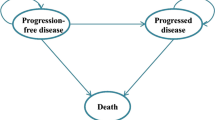
A cost-effectiveness analysis model for atezolizumab, including PD-L1-positive probability testing, was used to construct a partitioned survival model with three health states. To assess the robustness, a probabilistic sensitivity analysis (PSA) was conducted. The acceptable probability was defined as the probability of willingness-to-pay (WTP) over the incremental cost-effectiveness ratio (ICER). Multiple repetitions at WTP thresholds were calculated by continuously reducing the atezolizumab price.
Results
The ICER per quality-adjusted life year (QALY) for atezolizumab therapy only for patients with high PD-L1 expression compared to platinum-based chemotherapy for all patients was 31,975,792 yen per QALY. This is higher than the WTP threshold of 15,000,000 yen. If the cost of atezolizumab were reduced to 54% of the original cost (563,917 yen), the strategy of using atezolizumab for patients with high PD-L1 could become more cost-effective.
Conclusions
The results indicated that atezolizumab was not cost-effective compared to platinum-based chemotherapy as a first-line treatment for patients with unresectable advanced NSCLC. However, we suggest that the price of atezolizumab should be reduced to 54% of the original cost to meet the WTP threshold of 15,000,000 yen per QALY.





Similar content being viewed by others
References
Khajuria O, Sharma N. Epigenetic targeting for lung cancer treatment via CRISPR/Cas9 technology. Adv Cancer Biol Metastasis. 2021;3:100012.
Masters GA, Temin S, Azzoli CG, Giaccone G, Baker S Jr, Brahmer JR, et al. Systemic therapy for Stage IV non-small-cell lung cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2015;33:3488–515. https://doi.org/10.1200/JCO.2015.62.1342.
Gubens MA, Davies M. NCCN guidelines updates: new immunotherapy strategies for improving outcomes in non-small cell lung cancer. J Natl Compr Canc Netw. 2019;17(5.5):574–8. https://doi.org/10.6004/jnccn.2019.5005.
Peters S, Reck M, Smit EF, Mok T, Hellmann MD. How to make the best use of immunotherapy as first-line treatment of advanced/metastatic non-small-cell lung cancer. Ann Oncol. 2019;30:884–96. https://doi.org/10.1093/annonc/mdz109.
Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016;39:98–106. https://doi.org/10.1097/COC.0000000000000239.
Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–5. https://doi.org/10.1126/science.aar4060.
Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. 2020;383:1328–39. https://doi.org/10.1056/NEJMoa1917346.
Liu G, Kang S, Wang X, Shang F. Cost-effectiveness analysis of atezolizumab versus chemotherapy as first-line treatment for metastatic non-small-cell lung cancer with different PD-L1 expression status. Front Oncol. 2021;11: 669195. https://doi.org/10.3389/fonc.2021.669195.
Kang S, Wang X, Zhang Y, Zhang B, Shang F, Guo W. First-line treatments for extensive-stage small-cell lung cancer with immune checkpoint inhibitors plus chemotherapy: a network meta-analysis and cost-effectiveness analysis. Front Oncol. 2021;11: 740091. https://doi.org/10.3389/fonc.2021.740091.
Cheng S, Pei R, Li J, Li B, Tang L, Yin T, et al. Atezolizumab compared to chemotherapy for first-line treatment in non-small cell lung cancer with high PD-L1 expression: a cost-effectiveness analysis from US and Chinese perspectives. Ann Transl Med. 2021;9:1481. https://doi.org/10.2103/atm-21-4294.
Teng MM, Chen SY, Yang B, Wang Y, Han RY, An MN, et al. Determining the optimal PD-1/PD-L1 inhibitors for the first-line treatment of non-small-cell lung cancer with high-level PD-L1 expression in China. Cancer Med. 2021;10:6344–53. https://doi.org/10.1002/cam4.4191.
Fukuda T, Shiroiwa T. Application of economic evaluation of pharmaceuticals and medical devices in Japan. J Natl Inst Public Health. 2019;68:27–33.
Shiroiwa T, Fukuda T, Ikeda S, Takura T, Moriwaki K. Development of an official guideline for the economic evaluation of drugs/medical devices in Japan. Value Health. 2017;20:372–8. https://doi.org/10.1016/j.jval.2016.08.726.
Jassem J, de Marinis F, Giaccone G, Vergnenegre A, Barrios CH, Morise M, et al. Updated overall survival analysis from IMpower110: Atezolizumab versus platinum-based chemotherapy in treatment-naive programmed death-ligand 1-selected NSCLC. J Thorac Oncol. 2021;16:1872–82. https://doi.org/10.1016/j.jtho.2021.06.019.
Williams C, Lewsey JD, Mackay DF, Briggs AH. Estimation of survival probabilities for use in cost-effectiveness analyses: A comparison of a multi-state modeling survival analysis approach with partitioned survival and Markov decision-analytic modeling. Med Decis Making. 2017;37:427–39. https://doi.org/10.1177/0272989X16670617.
Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–65. https://doi.org/10.1016/S0140-6736(16)32517-X.
Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13:322–38. https://doi.org/10.1177/0272989X9301300409.
Japan nephrology s. [Special issue: Clinical practice guidebook for diagnosis and treatment of chronic kidney disease 2012]. Nihon Jinzo Gakkai Shi. 2012;54(8):1034-191.
Liu Q, Zhou Z, Luo X, Yi L, Peng L, Wan X, et al. First-line ICI monotherapies for advanced non-small-cell lung cancer patients with PD-L1 of at least 50%: a cost-effectiveness analysis. Front Pharmacol. 2021;12: 788569.
Nokihara H, Lu S, Mok TSK, Nakagawa K, Yamamoto N, Shi YK, et al. Randomized controlled trial of S-1 versus docetaxel in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy (East Asia S-1 Trial in Lung Cancer). Ann Oncol. 2017;28:2698–706. https://doi.org/10.1093/annonc/mdx419.
Cancer JJoL. Guidelines for Lung Cancer Treatment—including Malignant Pleural Mesothelioma and Thymic Tumors—2022 Edition: Kanehara; 2022.
Bow EJ, Rotstein C, Noskin GA, Laverdiere M, Schwarer AP, Segal BH, et al. A randomized, open-label, multicenter comparative study of the efficacy and safety of piperacillin-tazobactam and cefepime for the empirical treatment of febrile neutropenic episodes in patients with hematologic malignancies. Clin Infect Dis. 2006;43:447–59. https://doi.org/10.1086/505393.
Ohno S, Shoji A, Hatake K, Oya N, Igarashi A. Cost-effectiveness analysis of treatment regimens with obinutuzumab plus chemotherapy in Japan for untreated follicular lymphoma patients. J Med Econ. 2020;23:1130–41. https://doi.org/10.1080/13696998.2020.1791890.
Nafees B, Stafford M, Gavriel S, Bhalla S, Watkins J. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. 2008;6:84. https://doi.org/10.1186/1477-7525-6-84.
Nafees B, Lloyd AJ, Dewilde S, Rajan N, Lorenzo M. Health state utilities in non-small cell lung cancer: an international study. Asia Pac J Clin Oncol. 2017;13:e195–203. https://doi.org/10.1111/ajco.12477.
Handorf EA, McElligott S, Vachani A, Langer CJ, Bristol Demeter M, Armstrong K, et al. Cost effectiveness of personalized therapy for first-line treatment of stage IV and recurrent incurable adenocarcinoma of the lung. J Oncol Pract. 2012;8:267–74. https://doi.org/10.1200/JOP.2011.000502.
Westwood M, Joore M, Whiting P, van Asselt T, Ramaekers B, Armstrong N, et al. Epidermal growth factor receptor tyrosine kinase (EGFR-TK) mutation testing in adults with locally advanced or metastatic non-small cell lung cancer: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2014;18:1–166. https://doi.org/10.3310/hta18320.
Items and prices of drugs usable in insurance-covered healthcare; 2023; The Minister of Health, Labour and Welfare. https://www.mhlw.go.jp/topics/2022/04/tp20220401-01.html. 2023.
Tsukiyama I, Ejiri M, Yamamoto Y, Nakao H, Yoneda M, Matsuura K, et al. A cost-effectiveness analysis of gemcitabine plus cisplatin versus gemcitabine alone for treatment of advanced biliary tract cancer in Japan. J Gastrointest Cancer. 2017;48:326–32. https://doi.org/10.1007/s12029-016-9885-6.
Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Value Health. 2013;16:e1–5. https://doi.org/10.1016/j.jval.2013.02.010.
R Development Core Team. R: A Language and Environment for Statistical Computing; 2005. http://www.R-project.org. Vienna, Austria: R Foundation for Statistical Computing. p. 3–900051-07-0
OECD Data. "Exchange rates.": https://data.oecd.org/conversion/exchange-rates.htm. Accessed 15 Sep 2023.
Peng Y, Zeng X, Peng L, Liu Q, Yi L, Luo X, et al. First-line atezolizumab for metastatic NSCLC with high PD-L1 expression: a United States-based cost-effectiveness analysis. Adv Ther. 2021;38:2447–57. https://doi.org/10.1007/s12325-021-01734-6.
Chisaki Y, Kuwada Y, Matsumura C, Yano Y. Cost-effectiveness analysis of atezolizumab plus nab-paclitaxel for advanced PD-L1 positive triple-negative breast cancer in Japan. Clin Drug Investig. 2021;41:381–9. https://doi.org/10.1007/s40261-021-01017-6.
Su D, Wu B, Shi L. Cost-effectiveness of atezolizumab plus bevacizumab vs sorafenib as first-line treatment of unresectable hepatocellular carcinoma. JAMA Netw Open. 2021;4: e210037. https://doi.org/10.1001/jamanetworkopen.2021.0037.
Ionova Y, Vuong W, Sandoval O, Fong J, Vu V, Zhong L, et al. Cost-effectiveness analysis of atezolizumab versus durvalumab as first-line treatment of extensive-stage small-cell lung cancer in the USA. Clin Drug Investig. 2022;42:491–500. https://doi.org/10.1007/s40261-022-01157-3.
Ding D, Hu H, Liao M, Shi Y, She L, Yao L, et al. Cost-effectiveness analysis of atezolizumab plus chemotherapy in the first-line treatment of metastatic non-squamous non-small cell lung cancer. Adv Ther. 2020;37:2116–26. https://doi.org/10.1007/s12325-020-01292-3.
Acknowledgements
We would like to thank Editage (http://www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interests
The authors indicate no potential conflict of interest.
Availability of data and material
All data generated or analyzed during this study are included in this published article and the references.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Authors’ contributions
Yugo Chisaki: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data Curation, Writing—Original Draft, Visualization, Project administration, and Funding acquisition. Hajime Nakano: Investigation. Juna Minamide: Investigation. Yoshitaka Yano: Writing—Review & Editing, Supervision.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chisaki, Y., Nakano, H., Minamide, J. et al. Cost-Effectiveness Analysis of Atezolizumab versus Platinum-Based Chemotherapy as First-Line Treatment for Patients with Unresectable Advanced Non-small Cell Lung Cancer with PD-L1 Expression Status in Japan. Clin Drug Investig 43, 839–850 (2023). https://doi.org/10.1007/s40261-023-01311-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-023-01311-5