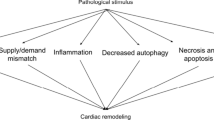
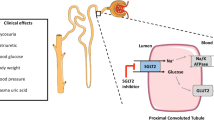
Abstract
Population aging combined with higher susceptibility to cardiovascular diseases in older adults is increasing the incidence of conditions such as atherosclerosis, myocardial infarction, heart failure, myocardial hypertrophy, myocardial fibrosis, arrhythmia, and hypertension. sodium–glucose cotransporter 2 inhibitors (SGLT2i) were originally developed as a novel oral drug for patients with type 2 diabetes mellitus. Unexpectedly, recent studies have shown that, beyond their effect on hyperglycemia, SGLT2i also have a variety of beneficial effects on cardiovascular disease. Experimental models of cardiovascular disease have shown that SGLT2i ameliorate the process of aging-related cardiovascular disease by inhibiting inflammation, reducing oxidative stress, and reversing endothelial dysfunction. In this review, we discuss the role of SGLT2i in aging-related cardiovascular disease and propose the use of SGLT2i to prevent and treat these conditions in older adults.





Similar content being viewed by others
References
Donato AJ, Machin DR, Lesniewski LA. Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circ Res. 2018;123:825–48.
Liu Z, Ma X, Ilyas I, Zheng X, Luo S, Little PJ, Kamato D, Sahebkar A, Wu W, Weng J, Xu S. Impact of sodium glucose cotransporter 2 (SGLT2) inhibitors on atherosclerosis: from pharmacology to pre-clinical and clinical therapeutics. Theranostics. 2021;11:4502–15.
Kulkarni AS, Aleksic S, Berger DM, Sierra F, Kuchel GA, Barzilai N. Geroscience-guided repurposing of FDA-approved drugs to target aging: a proposed process and prioritization. Aging Cell. 2022;21: e13596.
Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol. 2012;8:495–502.
Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia. 2017;60:215–25.
Wright EM, Turk E. The sodium/glucose cotransport family SLC5. Pflugers Arch. 2004;447:510–8.
Singh M, Kumar A. Risks associated with SGLT2 inhibitors: an overview. Curr Drug Saf. 2018;13:84–91.
Huang K, Luo X, Liao B, Li G, Feng J. Insights into SGLT2 inhibitor treatment of diabetic cardiomyopathy: focus on the mechanisms. Cardiovasc Diabetol. 2023;22:86.
Zelniker TA, Braunwald E. Mechanisms of cardiorenal effects of sodium-glucose cotransporter 2 inhibitors: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:422–34.
Monteiro P, Bergenstal RM, Toural E, Inzucchi SE, Zinman B, Hantel S, Kiš SG, Kaspers S, George JT, Fitchett D. Efficacy and safety of empagliflozin in older patients in the EMPA-REG OUTCOME® trial. Age Ageing. 2019;48:859–66.
Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado R, Bhatt DL, Leiter LA, McGuire DK, Wilding J, Sabatine MS. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–9.
Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D, Køber L, Petrie MC, McMurray J. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7:776–85.
Abdellatif M, Rainer PP, Sedej S, Kroemer G. Hallmarks of cardiovascular ageing. Nat Rev Cardiol. 2023;133:237–51
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–217.
Riera CE, Merkwirth C, De Magalhaes FC, Dillin A. Signaling networks determining life span. Annu Rev Biochem. 2016;85:35–64.
de Almeida A, Ribeiro TP, de Medeiros IA. Aging: molecular pathways and implications on the cardiovascular system. Oxid Med Cell Longev. 2017;2017:7941563.
Meschiari CA, Ero OK, Pan H, Finkel T, Lindsey ML. The impact of aging on cardiac extracellular matrix. Geroscience. 2017;39:7–18.
Uryga AK, Bennett MR. Ageing induced vascular smooth muscle cell senescence in atherosclerosis. J Physiol. 2016;594:2115–24.
Ingraham JP, Forbes ME, Riddle DR, Sonntag WE. Aging reduces hypoxia-induced microvascular growth in the rodent hippocampus. J Gerontol a Biol Sci Med Sci. 2008;63:12–20.
Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S4–9.
Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D, Abete P. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;13:757–72.
Picca A, Mankowski RT, Burman JL, Donisi L, Kim JS, Marzetti E, Leeuwenburgh C. Mitochondrial quality control mechanisms as molecular targets in cardiac ageing. Nat Rev Cardiol. 2018;15:543–54.
North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res. 2012;110:1097–108.
Fajemiroye JO, Da CL, Saavedra-Rodríguez R, Rodrigues KL, Naves LM, Mourão AA, Da SE, Williams N, Martins J, Sousa RB, Rebelo A, Reis A, Santos R, Ferreira-Neto ML, Pedrino GR. Aging-induced biological changes and cardiovascular diseases. Biomed Res Int. 2018;2018:7156435.
Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15:505–22.
Lakatta EG. Arterial aging is risky. J Appl Physiol. 1985;2008(105):1321–2.
Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997;82:291–5.
Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015;4:180–3.
Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–7.
Kwak HB. Aging, exercise, and extracellular matrix in the heart. J Exerc Rehabil. 2013;9:338–47.
Hipkiss AR. Mitochondrial dysfunction, proteotoxicity, and aging: causes or effects, and the possible impact of NAD+-controlled protein glycation. Adv Clin Chem. 2010;50:123–50.
Marchio P, Guerra-Ojeda S, Vila JM, Aldasoro M, Victor VM, Mauricio MD. Targeting early atherosclerosis: a focus on oxidative stress and inflammation. Oxid Med Cell Longev. 2019;2019:8563845.
Liu Y, Wang M, Liang Y, Wang C, Naruse K, Takahashi K. Treatment of oxidative stress with exosomes in myocardial ischemia. Int J Mol Sci. 2021;22:1729.
Misra MK, Sarwat M, Bhakuni P, Tuteja R, Tuteja N. Oxidative stress and ischemic myocardial syndromes. Med Sci Monit. 2009;15:A209–19.
Wang W, Kang PM. Oxidative stress and antioxidant treatments in cardiovascular diseases. Antioxidants (Basel). 2020;9:1292.
Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–4.
Holmström KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol. 2014;15:411–21.
Piquereau J, Caffin F, Novotova M, Lemaire C, Veksler V, Garnier A, Ventura-Clapier R, Joubert F. Mitochondrial dynamics in the adult cardiomyocytes: which roles for a highly specialized cell? Front Physiol. 2013;4:102.
Pagan LU, Gomes MJ, Gatto M, Mota G, Okoshi K, Okoshi MP. The role of oxidative stress in the aging heart. Antioxidants (Basel). 2022;11:336.
Daiber A, Di Lisa F, Oelze M, Kröller-Schön S, Steven S, Schulz E, Münzel T. Crosstalk of mitochondria with NADPH oxidase via reactive oxygen and nitrogen species signalling and its role for vascular function. Br J Pharmacol. 2017;174:1670–89.
Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med. 2011;51:1289–301.
Chandrasekaran A, Idelchik M, Melendez JA. Redox control of senescence and age-related disease. Redox Biol. 2017;11:91–102.
Van der Heiden K, Cuhlmann S, Luong LA, Zakkar M, Evans PC. Role of nuclear factor kappaB in cardiovascular health and disease. Clin Sci (Lond). 2010;118:593–605.
Ferrara N, Rinaldi B, Corbi G, Conti V, Stiuso P, Boccuti S, Rengo G, Rossi F, Filippelli A. Exercise training promotes SIRT1 activity in aged rats. Rejuvenation Res. 2008;11:139–50.
Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–66.
van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Lüscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–44.
Sun D, Huang A, Yan EH, Wu Z, Yan C, Kaminski PM, Oury TD, Wolin MS, Kaley G. Reduced release of nitric oxide to shear stress in mesenteric arteries of aged rats. Am J Physiol Heart Circ Physiol. 2004;286:H2249–56.
Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF. Superoxide excess in hypertension and aging: a common cause of endothelial dysfunction. Hypertension. 2001;37:529–34.
Francia P, Delli GC, Bachschmid M, Martin-Padura I, Savoia C, Migliaccio E, Pelicci PG, Schiavoni M, Lüscher TF, Volpe M, Cosentino F. Deletion of p66shc gene protects against age-related endothelial dysfunction. Circulation. 2004;110:2889–95.
Csiszar A, Labinskyy N, Orosz Z, Xiangmin Z, Buffenstein R, Ungvari Z. Vascular aging in the longest-living rodent, the naked mole rat. Am J Physiol Heart Circ Physiol. 2007;293:H919–27.
Ungvari Z, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith K, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37–47.
Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100:1659–66.
Jablonski KL, Seals DR, Eskurza I, Monahan KD, Donato AJ. High-dose ascorbic acid infusion abolishes chronic vasoconstriction and restores resting leg blood flow in healthy older men. J Appl Physiol. 1985;2007(103):1715–21.
Sepúlveda C, Palomo I, Fuentes E. Mechanisms of endothelial dysfunction during aging: predisposition to thrombosis. Mech Ageing Dev. 2017;164:91–9.
Ribeiro Acol TP. Cardiovascular effects induced by northeastern Brazilian red wine: role ofnitric oxide and redox sensitive pathways. J Funct Food. 2016;22:82–92.
Vanhoutte PM. Nitric oxide: from good to bad. Ann Vasc Dis. 2018;11:41–51.
Herrera MD, Mingorance C, Rodríguez-Rodríguez R, Alvarez DSM. Endothelial dysfunction and aging: an update. Ageing Res Rev. 2010;9:142–52.
Tesauro M, Mauriello A, Rovella V, Annicchiarico-Petruzzelli M, Cardillo C, Melino G, Di Daniele N. Arterial ageing: from endothelial dysfunction to vascular calcification. J Intern Med. 2017;281:471–82.
Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, Seals DR. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2009;297:H425–32.
Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW, Muller-Delp JM. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol. 2004;556:947–58.
Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol. 2009;587:3271–85.
Novella S, Dantas AP, Segarra G, Novensa L, Heras M, Hermenegildo C, Medina P. Aging enhances contraction to thromboxane A2 in aorta from female senescence-accelerated mice. Age (Dordrecht). 2013;35:117–28.
Cardillo C, Kilcoyne CM, Cannon RR, Panza JA. Interactions between nitric oxide and endothelin in the regulation of vascular tone of human resistance vessels in vivo. Hypertension. 2000;35:1237–41.
van der Bliek AM, Sedensky MM, Morgan PG. Cell biology of the mitochondrion. Genetics. 2017;207:843–71.
Dutta D, Calvani R, Bernabei R, Leeuwenburgh C, Marzetti E. Contribution of impaired mitochondrial autophagy to cardiac aging: mechanisms and therapeutic opportunities. Circ Res. 2012;110:1125–38.
Judge S, Leeuwenburgh C. Cardiac mitochondrial bioenergetics, oxidative stress, and aging. Am J Physiol Cell Physiol. 2007;292:C1983–92.
Lesnefsky EJ, Chen Q, Hoppel CL. Mitochondrial metabolism in aging heart. Circ Res. 2016;118:1593–611.
Dai DF, Rabinovitch PS, Ungvari Z. Mitochondria and cardiovascular aging. Circ Res. 2012;110:1109–24.
Passos JF, Saretzki G, Ahmed S, Nelson G, Richter T, Peters H, Wappler I, Birket MJ, Harold G, Schaeuble K, Birch-Machin MA, Kirkwood TB, von Zglinicki T. Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Biol. 2007;5: e110.
Karbowski M, Kurono C, Wozniak M, Ostrowski M, Teranishi M, Nishizawa Y, Usukura J, Soji T, Wakabayashi T. Free radical-induced megamitochondria formation and apoptosis. Free Radic Biol Med. 1999;26:396–409.
Sachs HG, Colgan JA, Lazarus ML. Ultrastructure of the aging myocardium: a morphometric approach. Am J Anat. 1977;150:63–71.
Judge S, Jang YM, Smith A, Hagen T, Leeuwenburgh C. Age-associated increases in oxidative stress and antioxidant enzyme activities in cardiac interfibrillar mitochondria: implications for the mitochondrial theory of aging. FASEB J. 2005;19:419–21.
Leeuwenburgh C, Wagner P, Holloszy JO, Sohal RS, Heinecke JW. Caloric restriction attenuates dityrosine cross-linking of cardiac and skeletal muscle proteins in aging mice. Arch Biochem Biophys. 1997;346:74–80.
Sohal RS, Ku HH, Agarwal S, Forster MJ, Lal H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech Ageing Dev. 1994;74:121–33.
Liu C, Bai J, Dan Q, Yang X, Lin K, Fu Z, Lu X, Xie X, Liu J, Fan L, Li Y, Ong S. Mitochondrial dysfunction contributes to aging-related atrial fibrillation. Oxid Med Cell Longev. 2021;2021:5530293.
Mohamed SA, Hanke T, Erasmi AW, Bechtel MJ, Scharfschwerdt M, Meissner C, Sievers HH, Gosslau A. Mitochondrial DNA deletions and the aging heart. Exp Gerontol. 2006;41:508–17.
Wanagat J, Cao Z, Pathare P, Aiken JM. Mitochondrial DNA deletion mutations colocalize with segmental electron transport system abnormalities, muscle fiber atrophy, fiber splitting, and oxidative damage in sarcopenia. FASEB J. 2001;15:322–32.
Khaidakov M, Heflich RH, Manjanatha MG, Myers MB, Aidoo A. Accumulation of point mutations in mitochondrial DNA of aging mice. Mutat Res. 2003;526:1–7.
Bose A, Beal MF. Mitochondrial dysfunction in Parkinson’s disease. J Neurochem. 2016;139(Suppl 1):216–31.
Yan C, Duanmu X, Zeng L, Liu B, Song Z. Mitochondrial DNA: distribution, mutations, and elimination. Cells. 2019;8:379.
Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–4.
Dai DF, Chen T, Wanagat J, Laflamme M, Marcinek DJ, Emond MJ, Ngo CP, Prolla TA, Rabinovitch PS. Age-dependent cardiomyopathy in mitochondrial mutator mice is attenuated by overexpression of catalase targeted to mitochondria. Aging Cell. 2010;9:536–44.
Lu T, Finkel T. Free radicals and senescence. Exp Cell Res. 2008;314:1918–22.
Csiszar A, Gautam T, Sosnowska D, Tarantini S, Banki E, Tucsek Z, Toth P, Losonczy G, Koller A, Reglodi D, Giles CB, Wren JD, Sonntag WE, Ungvari Z. Caloric restriction confers persistent anti-oxidative, pro-angiogenic, and anti-inflammatory effects and promotes anti-aging miRNA expression profile in cerebromicrovascular endothelial cells of aged rats. Am J Physiol Heart Circ Physiol. 2014;307:H292–306.
Springo Z, Tarantini S, Toth P, Tucsek Z, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates pressure-induced mitochondrial oxidative stress in mouse cerebral arteries. J Gerontol A Biol Sci Med Sci. 2015;70:1355–9.
Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Fulop GA, Hertelendy P, Gautam T, Farkas E, Perz A, Rabinovitch PS, Sonntag WE, Csiszar A, Ungvari Z. Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell. 2018;17:e12731.
Csiszar A, Sosnowska D, Wang M, Lakatta EG, Sonntag WE, Ungvari Z. Age-associated proinflammatory secretory phenotype in vascular smooth muscle cells from the non-human primate Macaca mulatta: reversal by resveratrol treatment. J Gerontol A Biol Sci Med Sci. 2012;67:811–20.
Rice KM, Preston DL, Walker EM, Blough ER. Aging influences multiple incidices of oxidative stress in the aortic media of the Fischer 344/NNiaxBrown Norway/BiNia rat. Free Radic Res. 2006;40:185–97.
Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–54.
Libby P. Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr. 2006;83:456S-460S.
de Almeida A, de Almeida RM, Dantas SH, de Lima SS, de Oliveira J, de Lourdes AADA, Alves R, de Menezes G, Dos SP, Gonçalves T, Schini-Kerth VB, de Medeiros IA. Unveiling the role of inflammation and oxidative stress on age-related cardiovascular diseases. Oxid Med Cell Longev. 2020;2020:1954398.
Latz E, Duewell P. NLRP3 inflammasome activation in inflammaging. Semin Immunol. 2018;40:61–73.
Gurung P, Lukens JR, Kanneganti TD. Mitochondria: diversity in the regulation of the NLRP3 inflammasome. Trends Mol Med. 2015;21:193–201.
Shi CS, Shenderov K, Huang NN, Kabat J, Abu-Asab M, Fitzgerald KA, Sher A, Kehrl JH. Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction. Nat Immunol. 2012;13:255–63.
Marín-Aguilar F, Lechuga-Vieco AV, Alcocer-Gómez E, Castejón-Vega B, Lucas J, Garrido C, Peralta-Garcia A, Pérez-Pulido AJ, Varela-López A, Quiles JL, Ryffel B, Flores I, Bullón P, Ruiz-Cabello J, Cordero MD. NLRP3 inflammasome suppression improves longevity and prevents cardiac aging in male mice. Aging Cell. 2020;19: e13050.
Santoro A, Ostan R, Candela M, Biagi E, Brigidi P, Capri M, Franceschi C. Gut microbiota changes in the extreme decades of human life: a focus on centenarians. Cell Mol Life Sci. 2018;75:129–48.
Man AL, Gicheva N, Nicoletti C. The impact of ageing on the intestinal epithelial barrier and immune system. Cell Immunol. 2014;289:112–8.
Takiishi T, Fenero C, Câmara N. Intestinal barrier and gut microbiota: shaping our immune responses throughout life. Tissue Barriers. 2017;5: e1373208.
Nagpal R, Mainali R, Ahmadi S, Wang S, Singh R, Kavanagh K, Kitzman DW, Kushugulova A, Marotta F, Yadav H. Gut microbiome and aging: physiological and mechanistic insights. Nutr Healthy Aging. 2018;4:267–85.
Kumar M, Babaei P, Ji B, Nielsen J. Human gut microbiota and healthy aging: recent developments and future prospective. Nutr Healthy Aging. 2016;4:3–16.
O’Brien M. The reciprocal relationship between inflammation and coagulation. Top Companion Anim Med. 2012;27:46–52.
Tzoran I, Hoffman R, Monreal M. Hemostasis and thrombosis in the oldest old. Semin Thromb Hemost. 2018;44:624–31.
Kreidy R. Influence of acquired and genetic risk factors on the prevention, management, and treatment of thromboembolic disease. Int J Vasc Med. 2014;2014: 859726.
Rea IM, Gibson DS, McGilligan V, McNerlan SE, Alexander HD, Ross OA. Age and age-related diseases: role of inflammation triggers and cytokines. Front Immunol. 2018;9:586.
Minciullo PL, Catalano A, Mandraffino G, Casciaro M, Crucitti A, Maltese G, Morabito N, Lasco A, Gangemi S, Basile G. Inflammaging and anti-inflammaging: the role of cytokines in extreme longevity. Arch Immunol Ther Exp (Warsz). 2016;64:111–26.
Banerjee S, Biehl A, Gadina M, Hasni S, Schwartz DM. JAK–STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs. 2017;77:521–46.
Frazier WJ, Xue J, Luce WA, Liu Y. MAPK signaling drives inflammation in LPS-stimulated cardiomyocytes: the route of crosstalk to G-protein-coupled receptors. PLoS ONE. 2012;7: e50071.
Jurk D, Wilson C, Passos JF, Oakley F, Correia-Melo C, Greaves L, Saretzki G, Fox C, Lawless C, Anderson R, Hewitt G, Pender SL, Fullard N, Nelson G, Mann J, van de Sluis B, Mann DA, von Zglinicki T. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat Commun. 2014;2:4172.
El AM, Angulo J, Vallejo S, Peiró C, Sánchez-Ferrer CF, Rodríguez-Mañas L. Mechanisms involved in the aging-induced vascular dysfunction. Front Physiol. 2012;3:132.
Taniguchi K, Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18:309–24.
Franceschi C, Garagnani P, Vitale G, Capri M, Salvioli S. Inflammaging and ‘garb-aging.’ Trends Endocrinol Metab. 2017;28:199–212.
Tilstra JS, Clauson CL, Niedernhofer LJ, Robbins PD. NF-κB in aging and disease. Aging Dis. 2011;2:449–65.
Lopez-Candales A, Hernández BP, Hernandez-Suarez DF, Harris D. Linking chronic inflammation with cardiovascular disease: from normal aging to the metabolic syndrome. J Nat Sci 2017;3(4):e341.
Maier HJ, Schips TG, Wietelmann A, Krüger M, Brunner C, Sauter M, Klingel K, Böttger T, Braun T, Wirth T. Cardiomyocyte-specific IκB kinase (IKK)/NF-κB activation induces reversible inflammatory cardiomyopathy and heart failure. Proc Natl Acad Sci USA. 2012;109:11794–9.
Hasegawa Y, Saito T, Ogihara T, Ishigaki Y, Yamada T, Imai J, Uno K, Gao J, Kaneko K, Shimosawa T, Asano T, Fujita T, Oka Y, Katagiri H. Blockade of the nuclear factor-κB pathway in the endothelium prevents insulin resistance and prolongs life spans. Circulation. 2012;125:1122–33.
Kubota T, McTiernan CF, Frye CS, Slawson SE, Lemster BH, Koretsky AP, Demetris AJ, Feldman AM. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-alpha. Circ Res. 1997;81:627–35.
Kalogeropoulos A, Georgiopoulou V, Psaty BM, Rodondi N, Smith AL, Harrison DG, Liu Y, Hoffmann U, Bauer DC, Newman AB, Kritchevsky SB, Harris TB, Butler J. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol. 2010;55:2129–37.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28.
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–57.
Wang JC, Bennett M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res. 2012;111:245–59.
Li B, Li W, Li X, Zhou H. Inflammation: a novel therapeutic target/direction in atherosclerosis. Curr Pharm Des. 2017;23:1216–27.
van der Vorst E, Daissormont I, Aslani M, Seijkens T, Wijnands E, Lutgens E, Duchene J, Santovito D, Döring Y, Halvorsen B, Aukrust P, Weber C, Höpken UE, Biessen E. Interruption of the CXCL13/CXCR5 chemokine axis enhances plasma IgM levels and attenuates atherosclerosis development. Thromb Haemost. 2020;120:344–7.
Asai K, Kudej RK, Shen YT, Yang GP, Takagi G, Kudej AB, Geng YJ, Sato N, Nazareno JB, Vatner DE, Natividad F, Bishop SP, Vatner SF. Peripheral vascular endothelial dysfunction and apoptosis in old monkeys. Arterioscler Thromb Vasc Biol. 2000;20:1493–9.
Donato AJ, Magerko KA, Lawson BR, Durrant JR, Lesniewski LA, Seals DR. SIRT-1 and vascular endothelial dysfunction with ageing in mice and humans. J Physiol. 2011;589:4545–54.
Gössl M, Yoon MH, Choi BJ, Rihal C, Tilford JM, Reriani M, Gulati R, Sandhu G, Eeckhout E, Lennon R, Lerman LO, Lerman A. Accelerated coronary plaque progression and endothelial dysfunction: serial volumetric evaluation by IVUS. JACC Cardiovasc Imaging. 2014;7:103–4.
Widlansky ME, Gokce N, Keaney JJ, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–60.
Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363–8.
Osorio H, Coronel I, Arellano A, Pacheco U, Bautista R, Franco M, Escalante B. Sodium-glucose cotransporter inhibition prevents oxidative stress in the kidney of diabetic rats. Oxid Med Cell Longev. 2012;2012: 542042.
Tahara A, Kurosaki E, Yokono M, Yamajuku D, Kihara R, Hayashizaki Y, Takasu T, Imamura M, Li Q, Tomiyama H, Kobayashi Y, Noda A, Sasamata M, Shibasaki M. Effects of SGLT2 selective inhibitor ipragliflozin on hyperglycemia, hyperlipidemia, hepatic steatosis, oxidative stress, inflammation, and obesity in type 2 diabetic mice. Eur J Pharmacol. 2013;715:246–55.
Lin B, Koibuchi N, Hasegawa Y, Sueta D, Toyama K, Uekawa K, Ma M, Nakagawa T, Kusaka H, Kim-Mitsuyama S. Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc Diabetol. 2014;13:148.
Park SH, Farooq MA, Gaertner S, Bruckert C, Qureshi AW, Lee HH, Benrahla D, Pollet B, Stephan D, Ohlmann P, Lessinger JM, Mayoux E, Auger C, Morel O, Schini-Kerth VB. Empagliflozin improved systolic blood pressure, endothelial dysfunction and heart remodeling in the metabolic syndrome ZSF1 rat. Cardiovasc Diabetol. 2020;19:19.
Salim HM, Fukuda D, Yagi S, Soeki T, Shimabukuro M, Sata M. Glycemic control with ipragliflozin, a novel selective SGLT2 inhibitor, ameliorated endothelial dysfunction in streptozotocin-induced diabetic mouse. Front Cardiovasc Med. 2016;3:43.
Gaspari T, Spizzo I, Liu H, Hu Y, Simpson RW, Widdop RE, Dear AE. Dapagliflozin attenuates human vascular endothelial cell activation and induces vasorelaxation: a potential mechanism for inhibition of atherogenesis. Diab Vasc Dis Res. 2018;15:64–73.
Lee DM, Battson ML, Jarrell DK, Hou S, Ecton KE, Weir TL, Gentile CL. SGLT2 inhibition via dapagliflozin improves generalized vascular dysfunction and alters the gut microbiota in type 2 diabetic mice. Cardiovasc Diabetol. 2018;17:62.
Sposito AC, Breder I, Soares A, Kimura-Medorima ST, Munhoz DB, Cintra R, Bonilha I, Oliveira DC, Breder JC, Cavalcante P, Moreira C, Moura FA, de Lima-Junior JC, Do CH, Barreto J, Nadruz W, Carvalho L, Quinaglia T. Dapagliflozin effect on endothelial dysfunction in diabetic patients with atherosclerotic disease: a randomized active-controlled trial. Cardiovasc Diabetol. 2021;20:74.
Rahadian A, Fukuda D, Salim HM, Yagi S, Kusunose K, Yamada H, Soeki T, Sata M. Canagliflozin prevents diabetes-induced vascular dysfunction in ApoE-deficient mice. J Atheroscler Thromb. 2020;27:1141–51.
Uthman L, Homayr A, Juni RP, Spin EL, Kerindongo R, Boomsma M, Hollmann MW, Preckel B, Koolwijk P, van Hinsbergh V, Zuurbier CJ, Albrecht M, Weber NC. Empagliflozin and dapagliflozin reduce ROS generation and restore NO bioavailability in tumor necrosis factor α-stimulated human coronary arterial endothelial cells. Cell Physiol Biochem. 2019;53:865–86.
Ganz P, Vita JA. Testing endothelial vasomotor function: nitric oxide, a multipotent molecule. Circulation. 2003;108:2049–53.
Moncada S, Higgs A. The l-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–12.
Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–42.
El-Daly M, Pulakazhi VV, Saifeddine M, Mihara K, Kang S, Fedak P, Alston LA, Hirota SA, Ding H, Triggle CR, Hollenberg MD. Hyperglycaemic impairment of PAR2-mediated vasodilation: prevention by inhibition of aortic endothelial sodium-glucose-co-transporter-2 and minimizing oxidative stress. Vasc Pharmacol. 2018;109:56–71.
Lambadiari V, Thymis J, Kouretas D, Skaperda Z, Tekos F, Kousathana F, Kountouri A, Balampanis K, Parissis J, Andreadou I, Tsoumani M, Chania C, Katogiannis K, Dimitriadis G, Bamias A, Ikonomidis I. Effects of a 12-month treatment with glucagon-like peptide-1 receptor agonists, sodium-glucose cotransporter-2 inhibitors, and their combination on oxidant and antioxidant biomarkers in patients with type 2 diabetes. Antioxidants (Basel). 2021;10:1379.
Khemais-Benkhiat S, Belcastro E, Idris-Khodja N, Park SH, Amoura L, Abbas M, Auger C, Kessler L, Mayoux E, Toti F, Schini-Kerth VB. Angiotensin II-induced redox-sensitive SGLT1 and 2 expression promotes high glucose-induced endothelial cell senescence. J Cell Mol Med. 2020;24:2109–22.
Grossin N, Auger F, Niquet-Leridon C, Durieux N, Montaigne D, Schmidt AM, Susen S, Jacolot P, Beuscart JB, Tessier FJ, Boulanger E. Dietary CML-enriched protein induces functional arterial aging in a RAGE-dependent manner in mice. Mol Nutr Food Res. 2015;59:927–38.
Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog. 2006;5:14.
Shanmugam N, Figarola JL, Li Y, Swiderski PM, Rahbar S, Natarajan R. Proinflammatory effects of advanced lipoxidation end products in monocytes. Diabetes. 2008;57:879–88.
Zhang J, Shao S, Han D, Xu Y, Jiao D, Wu J, Yang F, Ge Y, Shi S, Li Y, Wen W, Qin W. High mobility group box 1 promotes the epithelial-to-mesenchymal transition in prostate cancer PC3 cells via the RAGE/NF-κB signaling pathway. Int J Oncol. 2018;53:659–71.
Ahmad S, Siddiqui Z, Rehman S, Khan MY, Khan H, Khanum S, Alouffi S, Saeed M. A glycation angle to look into the diabetic vasculopathy: cause and cure. Curr Vasc Pharmacol. 2017;15:352–64.
Oelze M, Kröller-Schön S, Welschof P, Jansen T, Hausding M, Mikhed Y, Stamm P, Mader M, Zinßius E, Agdauletova S, Gottschlich A, Steven S, Schulz E, Bottari SP, Mayoux E, Münzel T, Daiber A. The sodium-glucose co-transporter 2 inhibitor empagliflozin improves diabetes-induced vascular dysfunction in the streptozotocin diabetes rat model by interfering with oxidative stress and glucotoxicity. PLoS ONE. 2014;9: e112394.
Leng W, Ouyang X, Lei X, Wu M, Chen L, Wu Q, Deng W, Liang Z. The SGLT-2 inhibitor dapagliflozin has a therapeutic effect on atherosclerosis in diabetic ApoE(−/−) mice. Mediators Inflamm. 2016;2016:6305735.
Nakatsu Y, Kokubo H, Bumdelger B, Yoshizumi M, Yamamotoya T, Matsunaga Y, Ueda K, Inoue Y, Inoue MK, Fujishiro M, Kushiyama A, Ono H, Sakoda H, Asano T. The SGLT2 inhibitor luseogliflozin rapidly normalizes aortic mRNA levels of inflammation-related but not lipid-metabolism-related genes and suppresses atherosclerosis in diabetic ApoE KO mice. Int J Mol Sci. 2017;18(8):E1704.
Dimitriadis GK, Nasiri-Ansari N, Agrogiannis G, Kostakis ID, Randeva MS, Nikiteas N, Patel VH, Kaltsas G, Papavassiliou AG, Randeva HS, Kassi E. Empagliflozin improves primary haemodynamic parameters and attenuates the development of atherosclerosis in high fat diet fed APOE knockout mice. Mol Cell Endocrinol. 2019;494: 110487.
Han JH, Oh TJ, Lee G, Maeng HJ, Lee DH, Kim KM, Choi SH, Jang HC, Lee HS, Park KS, Kim YB, Lim S. The beneficial effects of empagliflozin, an SGLT2 inhibitor, on atherosclerosis in ApoE(−/−) mice fed a western diet. Diabetologia. 2017;60:364–76.
D’Onofrio N, Sardu C, Trotta MC, Scisciola L, Turriziani F, Ferraraccio F, Panarese I, Petrella L, Fanelli M, Modugno P, Massetti M, Marfella LV, Sasso FC, Rizzo MR, Barbieri M, Furbatto F, Minicucci F, Mauro C, Federici M, Balestrieri ML, Paolisso G, Marfella R. Sodium-glucose co-transporter2 expression and inflammatory activity in diabetic atherosclerotic plaques: effects of sodium-glucose co-transporter2 inhibitor treatment. Mol Metab. 2021;54: 101337.
Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–61.
Van Tassell BW, Toldo S, Mezzaroma E, Abbate A. Targeting interleukin-1 in heart disease. Circulation. 2013;128:1910–23.
Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–87.
Byrne NJ, Matsumura N, Maayah ZH, Ferdaoussi M, Takahara S, Darwesh AM, Levasseur JL, Jahng J, Vos D, Parajuli N, El-Kadi A, Braam B, Young ME, Verma S, Light PE, Sweeney G, Seubert JM, Dyck J. Empagliflozin blunts worsening cardiac dysfunction associated with reduced NLRP3 (nucleotide-binding domain-like receptor protein 3) inflammasome activation in heart failure. Circ Heart Fail. 2020;13: e6277.
Kim SR, Lee SG, Kim SH, Kim JH, Choi E, Cho W, Rim JH, Hwang I, Lee CJ, Lee M, Oh CM, Jeon JY, Gee HY, Kim JH, Lee BW, Kang ES, Cha BS, Lee MS, Yu JW, Cho JW, Kim JS, Lee YH. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat Commun. 2020;11:2127.
Min SH, Oh TJ, Baek SI, Lee DH, Kim KM, Moon JH, Choi SH, Park KS, Jang HC, Lim S. Degree of ketonaemia and its association with insulin resistance after dapagliflozin treatment in type 2 diabetes. Diabetes Metab. 2018;44:73–6.
Ye Y, Bajaj M, Yang HC, Perez-Polo JR, Birnbaum Y. SGLT-2 inhibition with dapagliflozin reduces the activation of the Nlrp3/ASC inflammasome and attenuates the development of diabetic cardiomyopathy in mice with type 2 diabetes. Further augmentation of the effects with saxagliptin, a DPP4 inhibitor. Cardiovasc Drugs Ther. 2017;31:119–32.
Chen H, Tran D, Yang HC, Nylander S, Birnbaum Y, Ye Y. Dapagliflozin and ticagrelor have additive effects on the attenuation of the activation of the NLRP3 inflammasome and the progression of diabetic cardiomyopathy: an AMPK-mTOR interplay. Cardiovasc Drugs Ther. 2020;34:443–61.
Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–81.
Lüscher TF, Creager MA, Beckman JA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part II. Circulation. 2003;108:1655–61.
Behnammanesh G, Durante GL, Khanna YP, Peyton KJ, Durante W. Canagliflozin inhibits vascular smooth muscle cell proliferation and migration: role of heme oxygenase-1. Redox Biol. 2020;32: 101527.
Takahashi H, Nomiyama T, Terawaki Y, Horikawa T, Kawanami T, Hamaguchi Y, Tanaka T, Motonaga R, Fukuda T, Tanabe M, Yanase T. Combined treatment with DPP-4 inhibitor linagliptin and SGLT2 inhibitor empagliflozin attenuates neointima formation after vascular injury in diabetic mice. Biochem Biophys Rep. 2019;18: 100640.
Tian K, Xu Y, Sahebkar A, Xu S. CD36 in atherosclerosis: pathophysiological mechanisms and therapeutic implications. Curr Atheroscler Rep. 2020;22:59.
Pennig J, Scherrer P, Gissler MC, Anto-Michel N, Hoppe N, Füner L, Härdtner C, Stachon P, Wolf D, Hilgendorf I, Mullick A, Bode C, Zirlik A, Goldberg IJ, Willecke F. Glucose lowering by SGLT2-inhibitor empagliflozin accelerates atherosclerosis regression in hyperglycemic STZ-diabetic mice. Sci Rep. 2019;9:17937.
Terasaki M, Hiromura M, Mori Y, Kohashi K, Nagashima M, Kushima H, Watanabe T, Hirano T. Amelioration of hyperglycemia with a sodium-glucose cotransporter 2 inhibitor prevents macrophage-driven atherosclerosis through macrophage foam cell formation suppression in type 1 and type 2 diabetic mice. PLoS ONE. 2015;10: e143396.
Koyani CN, Plastira I, Sourij H, Hallström S, Schmidt A, Rainer PP, Bugger H, Frank S, Malle E, von Lewinski D. Empagliflozin protects heart from inflammation and energy depletion via AMPK activation. Pharmacol Res. 2020;158: 104870.
Lee TM, Chang NC, Lin SZ. Dapagliflozin, a selective SGLT2 inhibitor, attenuated cardiac fibrosis by regulating the macrophage polarization via STAT3 signaling in infarcted rat hearts. Free Radic Biol Med. 2017;104:298–310.
Lee SG, Lee SJ, Lee JJ, Kim JS, Lee OH, Kim CK, Kim D, Lee YH, Oh J, Park S, Jeon OH, Hong SJ, Ahn CM, Kim BK, Ko YG, Choi D, Hong MK, Jang Y. Anti-inflammatory effect for atherosclerosis progression by sodium-glucose cotransporter 2 (SGLT-2) inhibitor in a normoglycemic rabbit model. Korean Circ J. 2020;50:443–57.
Wu MD, Atkinson TM, Lindner JR. Platelets and von Willebrand factor in atherogenesis. Blood. 2017;129:1415–9.
Kraakman MJ, Lee MK, Al-Sharea A, Dragoljevic D, Barrett TJ, Montenont E, Basu D, Heywood S, Kammoun HL, Flynn M, Whillas A, Hanssen NM, Febbraio MA, Westein E, Fisher EA, Chin-Dusting J, Cooper ME, Berger JS, Goldberg IJ, Nagareddy PR, Murphy AJ. Neutrophil-derived S100 calcium-binding proteins A8/A9 promote reticulated thrombocytosis and atherogenesis in diabetes. J Clin Investig. 2017;127:2133–47.
Spigoni V, Fantuzzi F, Carubbi C, Pozzi G, Masselli E, Gobbi G, Solini A, Bonadonna RC, Dei CA. Sodium-glucose cotransporter 2 inhibitors antagonize lipotoxicity in human myeloid angiogenic cells and ADP-dependent activation in human platelets: potential relevance to prevention of cardiovascular events. Cardiovasc Diabetol. 2020;19:46.
Sakurai S, Jojima T, Iijima T, Tomaru T, Usui I, Aso Y. Empagliflozin decreases the plasma concentration of plasminogen activator inhibitor-1 (PAI-1) in patients with type 2 diabetes: association with improvement of fibrinolysis. J Diabetes Complications. 2020;34: 107703.
Kohlmorgen C, Gerfer S, Feldmann K, Twarock S, Hartwig S, Lehr S, Klier M, Krüger I, Helten C, Keul P, Kahl S, Polzin A, Elvers M, Flögel U, Kelm M, Levkau B, Roden M, Fischer JW, Grandoch M. Dapagliflozin reduces thrombin generation and platelet activation: implications for cardiovascular risk reduction in type 2 diabetes mellitus. Diabetologia. 2021;64:1834–49.
Kempf T, Wollert KC. Iron and atherosclerosis: too much of a good thing can be bad. Eur Heart J. 2020;41:2696–8.
Vinchi F, Porto G, Simmelbauer A, Altamura S, Passos ST, Garbowski M, Silva A, Spaich S, Seide SE, Sparla R, Hentze MW, Muckenthaler MU. Atherosclerosis is aggravated by iron overload and ameliorated by dietary and pharmacological iron restriction. Eur Heart J. 2020;41:2681–95.
You SA, Wang Q. Ferritin in atherosclerosis. Clin Chim Acta. 2005;357:1–16.
Xu S. Iron and atherosclerosis: the link revisited. Trends Mol Med. 2019;25:659–61.
Ferrannini E, Murthy AC, Lee YH, Muscelli E, Weiss S, Ostroff RM, Sattar N, Williams SA, Ganz P. Mechanisms of sodium-glucose cotransporter 2 inhibition: insights from large-scale proteomics. Diabetes Care. 2020;43:2183–9.
Ghanim H, Abuaysheh S, Hejna J, Green K, Batra M, Makdissi A, Chaudhuri A, Dandona P. Dapagliflozin suppresses hepcidin and increases erythropoiesis. J Clin Endocrinol Metab. 2020;105:e1056–63.
Liao X, Sluimer JC, Wang Y, Subramanian M, Brown K, Pattison JS, Robbins J, Martinez J, Tabas I. Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab. 2012;15:545–53.
Li Y, Chen Y. AMPK and autophagy. Adv Exp Med Biol. 2019;1206:85–108.
Xu C, Wang W, Zhong J, Lei F, Xu N, Zhang Y, Xie W. Canagliflozin exerts anti-inflammatory effects by inhibiting intracellular glucose metabolism and promoting autophagy in immune cells. Biochem Pharmacol. 2018;152:45–59.
Aragón-Herrera A, Feijóo-Bandín S, Otero SM, Barral L, Campos-Toimil M, Gil-Longo J, Costa PT, García-Caballero T, Rodríguez-Segade S, Rodríguez J, Tarazón E, Roselló-Lletí E, Portolés M, Gualillo O, González-Juanatey JR, Lago F. Empagliflozin reduces the levels of CD36 and cardiotoxic lipids while improving autophagy in the hearts of Zucker diabetic fatty rats. Biochem Pharmacol. 2019;170: 113677.
Luo G, Jian Z, Zhu Y, Zhu Y, Chen B, Ma R, Tang F, Xiao Y. Sirt1 promotes autophagy and inhibits apoptosis to protect cardiomyocytes from hypoxic stress. Int J Mol Med. 2019;43:2033–43.
Umino H, Hasegawa K, Minakuchi H, Muraoka H, Kawaguchi T, Kanda T, Tokuyama H, Wakino S, Itoh H. High basolateral glucose increases sodium-glucose cotransporter 2 and reduces sirtuin-1 in renal tubules through glucose transporter-2 detection. Sci Rep. 2018;8:6791.
Wang CY, Chen CC, Lin MH, Su HT, Ho MY, Yeh JK, Tsai ML, Hsieh IC, Wen MS. TLR9 binding to beclin 1 and mitochondrial sirt3 by a sodium-glucose co-transporter 2 inhibitor protects the heart from doxorubicin toxicity. Biology (Basel). 2020;9(11):369.
Hurtubise J, McLellan K, Durr K, Onasanya O, Nwabuko D, Ndisang JF. The different facets of dyslipidemia and hypertension in atherosclerosis. Curr Atheroscler Rep. 2016;18:82.
Nasiri-Ansari Ν, Dimitriadis GK, Agrogiannis G, Perrea D, Kostakis ID, Kaltsas G, Papavassiliou AG, Randeva HS, Kassi E. Canagliflozin attenuates the progression of atherosclerosis and inflammation process in APOE knockout mice. Cardiovasc Diabetol. 2018;17:106.
Liu Y, Xu J, Wu M, Xu B, Kang L. Empagliflozin protects against atherosclerosis progression by modulating lipid profiles and sympathetic activity. Lipids Health Dis. 2021;20:5.
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–292.
Shih H, Lee B, Lee RJ, Boyle AJ. The aging heart and post-infarction left ventricular remodeling. J Am Coll Cardiol. 2011;57:9–17.
Abdellatif M, Sedej S, Carmona-Gutierrez D, Madeo F, Kroemer G. Autophagy in cardiovascular aging. Circ Res. 2018;123:803–24.
de Almeida AJPO, de Almeida Rezende MS, Dantas SH, de Lima Silva S, de Oliveira JCPL, de Lourdes Assunção Araújo De Azevedo F, Alves RMFR, de Menezes GMS, Dos Santos PF, Gonçalves TAF, Schini-Kerth VB, de Medeiros IA. Unveiling the role of inflammation and oxidative stress on age-related cardiovascular diseases. Oxid Med Cell Longev. 2020;2020:1–20.
Kates AM, Herrero P, Dence C, Soto P, Srinivasan M, Delano DG, Ehsani A, Gropler RJ. Impact of aging on substrate metabolism by the human heart. J Am Coll Cardiol. 2003;41:293–9.
Liu Y, Wu M, Xu J, Xu B, Kang L. Empagliflozin prevents from early cardiac injury post myocardial infarction in non-diabetic mice. Eur J Pharm Sci. 2021;161: 105788.
Lee SY, Lee TW, Park GT, Kim JH, Lee HC, Han JH, Yoon A, Yoon D, Kim S, Jung SM, Choi JH, Chon MK, Lee SH, Hwang KW, Kim J, Park YH, Kim JH, Chun KJ, Hur J. Sodium/glucose co-transporter 2 inhibitor, empagliflozin, alleviated transient expression of sglt2 after myocardial infarction. Korean Circ J. 2021;51:251–62.
Lim VG, Bell RM, Arjun S, Kolatsi-Joannou M, Long DA, Yellon DM. SGLT2 inhibitor, canagliflozin, attenuates myocardial infarction in the diabetic and nondiabetic heart. JACC Basic Transl Sci. 2019;4:15–26.
Sayour AA, Korkmaz-Icöz S, Loganathan S, Ruppert M, Sayour VN, Oláh A, Benke K, Brune M, Benkő R, Horváth EM, Karck M, Merkely B, Radovits T, Szabó G. Acute canagliflozin treatment protects against in vivo myocardial ischemia–reperfusion injury in non-diabetic male rats and enhances endothelium-dependent vasorelaxation. J Transl Med. 2019;17:127.
Santos-Gallego CG, Requena-Ibanez JA, San AR, Ishikawa K, Watanabe S, Picatoste B, Flores E, Garcia-Ropero A, Sanz J, Hajjar RJ, Fuster V, Badimon JJ. Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. J Am Coll Cardiol. 2019;73:1931–44.
Uthman L, Nederlof R, Eerbeek O, Baartscheer A, Schumacher C, Buchholtz N, Hollmann MW, Coronel R, Weber NC, Zuurbier CJ. Delayed ischaemic contracture onset by empagliflozin associates with NHE1 inhibition and is dependent on insulin in isolated mouse hearts. Cardiovasc Res. 2019;115:1533–45.
Jiang K, Xu Y, Wang D, Chen F, Tu Z, Qian J, Xu S, Xu Y, Hwa J, Li J, Shang H, Xiang Y. Cardioprotective mechanism of SGLT2 inhibitor against myocardial infarction is through reduction of autosis. Protein Cell. 2022;13:336–59.
Oshima H, Miki T, Kuno A, Mizuno M, Sato T, Tanno M, Yano T, Nakata K, Kimura Y, Abe K, Ohwada W, Miura T. Empagliflozin, an SGLT2 inhibitor, reduced the mortality rate after acute myocardial infarction with modification of cardiac metabolomes and antioxidants in diabetic rats. J Pharmacol Exp Ther. 2019;368:524–34.
Díez-Villanueva P, Alfonso F. Heart failure in the elderly. J Geriatr Cardiol. 2016;13:115–7.
Lazzarini V, Mentz RJ, Fiuzat M, Metra M, O’Connor CM. Heart failure in elderly patients: distinctive features and unresolved issues. Eur J Heart Fail. 2013;15:717–23.
Mirza M, Strunets A, Shen WK, Jahangir A. Mechanisms of arrhythmias and conduction disorders in older adults. Clin Geriatr Med. 2012;28:555–73.
Strait JB, Lakatta EG. Aging-associated cardiovascular changes and their relationship to heart failure. Heart Fail Clin. 2012;8:143–64.
Kario K, Okada K, Kato M, Nishizawa M, Yoshida T, Asano T, Uchiyama K, Niijima Y, Katsuya T, Urata H, Osuga JI, Fujiwara T, Yamazaki S, Tomitani N, Kanegae H. 24-hour blood pressure-lowering effect of an SGLT-2 inhibitor in patients with diabetes and uncontrolled nocturnal hypertension: results from the randomized, placebo-controlled SACRA study. Circulation. 2018;139:2089–97.
Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding J, Ruff CT, Gause-Nilsson I, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–57.
Maruyama T, Takashima H, Oguma H, Nakamura Y, Ohno M, Utsunomiya K, Furukawa T, Tei R, Abe M. Canagliflozin improves erythropoiesis in diabetes patients with anemia of chronic kidney disease. Diabetes Technol Ther. 2019;21:713–20.
Mustroph J, Wagemann O, Lücht CM, Trum M, Hammer KP, Sag CM, Lebek S, Tarnowski D, Reinders J, Perbellini F, Terracciano C, Schmid C, Schopka S, Hilker M, Zausig Y, Pabel S, Sossalla ST, Schweda F, Maier LS, Wagner S. Empagliflozin reduces Ca/calmodulin-dependent kinase II activity in isolated ventricular cardiomyocytes. Esc Heart Fail. 2018;5:642–8.
Uthman L, Baartscheer A, Bleijlevens B, Schumacher CA, Fiolet J, Koeman A, Jancev M, Hollmann MW, Weber NC, Coronel R, Zuurbier CJ. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na(+)/H(+) exchanger, lowering of cytosolic Na(+) and vasodilation. Diabetologia. 2018;61:722–6.
Gerstenblith G, Frederiksen J, Yin FC, Fortuin NJ, Lakatta EG, Weisfeldt ML. Echocardiographic assessment of a normal adult aging population. Circulation. 1977;56:273–8.
Olivetti G, Melissari M, Capasso JM, Anversa P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ Res. 1991;68:1560–8.
Fleg JL, O’Connor F, Gerstenblith G, Becker LC, Clulow J, Schulman SP, Lakatta EG. Impact of age on the cardiovascular response to dynamic upright exercise in healthy men and women. J Appl Physiol. 1985;1995(78):890–900.
Kimura K, Ieda M, Kanazawa H, Yagi T, Tsunoda M, Ninomiya S, Kurosawa H, Yoshimi K, Mochizuki H, Yamazaki K, Ogawa S, Fukuda K. Cardiac sympathetic rejuvenation: a link between nerve function and cardiac hypertrophy. Circ Res. 2007;100:1755–64.
de Lucia C, Eguchi A, Koch WJ. New insights in cardiac β-adrenergic signaling during heart failure and aging. Front Pharmacol. 2018;9:904.
Xu L, Brink M. mTOR, cardiomyocytes and inflammation in cardiac hypertrophy. Biochim Biophys Acta. 2016;1863:1894–903.
Zhang N, Feng B, Ma X, Sun K, Xu G, Zhou Y. Dapagliflozin improves left ventricular remodeling and aorta sympathetic tone in a pig model of heart failure with preserved ejection fraction. Cardiovasc Diabetol. 2019;18:107.
Kopp C, Linz P, Dahlmann A, Hammon M, Jantsch J, Müller DN, Schmieder RE, Cavallaro A, Eckardt KU, Uder M, Luft FC, Titze J. 23Na magnetic resonance imaging-determined tissue sodium in healthy subjects and hypertensive patients. Hypertension. 2013;61:635–40.
Schneider MP, Raff U, Kopp C, Scheppach JB, Toncar S, Wanner C, Schlieper G, Saritas T, Floege J, Schmid M, Birukov A, Dahlmann A, Linz P, Janka R, Uder M, Schmieder RE, Titze JM, Eckardt KU. Skin sodium concentration correlates with left ventricular hypertrophy in CKD. J Am Soc Nephrol. 2017;28:1867–76.
Karg MV, Bosch A, Kannenkeril D, Striepe K, Ott C, Schneider MP, Boemke-Zelch F, Linz P, Nagel AM, Titze J, Uder M, Schmieder RE. SGLT-2-inhibition with dapagliflozin reduces tissue sodium content: a randomised controlled trial. Cardiovasc Diabetol. 2018;17:5.
Abdul KA, Clarke K, Evans RD. Cardiac ketone body metabolism. Biochim Biophys Acta Mol Basis Dis. 2020;1866: 165739.
Sithara T, Drosatos K. Metabolic complications in cardiac aging. Front Physiol. 2021;12: 669497.
Lehrke M. SGLT2 inhibition: changing what fuels the heart. J Am Coll Cardiol. 2019;73:1945–7.
Asensio LM, Lax A, Hernandez VA, Saura GE, Hernandez-Martinez A, Fernandez DPM, Bayes-Genis A, Pascual FD. Empagliflozin improves post-infarction cardiac remodeling through GTP enzyme cyclohydrolase 1 and irrespective of diabetes status. Sci Rep. 2020;10:13553.
Lv J, Chen J, Wang M, Yan F. Klotho alleviates indoxyl sulfate-induced heart failure and kidney damage by promoting M2 macrophage polarization. Aging (Albany NY). 2020;12:9139–50.
Xu L, Nagata N, Nagashimada M, Zhuge F, Ni Y, Chen G, Mayoux E, Kaneko S, Ota T. SGLT2 inhibition by empagliflozin promotes fat utilization and browning and attenuates inflammation and insulin resistance by polarizing M2 macrophages in diet-induced obese mice. EBioMedicine. 2017;20:137–49.
Burkauskiene A. Age-related changes in the structure of myocardial collagen network of auricle of the right atrium in healthy persons and ischemic heart disease patients. Medicina (Kaunas). 2005;41:145–54.
Park S, Nguyen NB, Pezhouman A, Ardehali R. Cardiac fibrosis: potential therapeutic targets. Transl Res. 2019;209:121–37.
Steinhauser ML, Lee RT. Regeneration of the heart. EMBO Mol Med. 2011;3:701–12.
Requena-Ibáñez JA, Santos-Gallego CG, Rodriguez-Cordero A, Vargas-Delgado AP, Mancini D, Sartori S, Atallah-Lajam F, Giannarelli C, Macaluso F, Lala A, Sanz J, Fuster V, Badimon JJ. Mechanistic insights of empagliflozin in nondiabetic patients with HFrEF: from the EMPA-TROPISM study. JACC Heart Fail. 2021;9:578–89.
Arow M, Waldman M, Yadin D, Nudelman V, Shainberg A, Abraham NG, Freimark D, Kornowski R, Aravot D, Hochhauser E, Arad M. Sodium-glucose cotransporter 2 inhibitor Dapagliflozin attenuates diabetic cardiomyopathy. Cardiovasc Diabetol. 2020;19:7.
Moore EE, Jefferson AL. Impact of cardiovascular hemodynamics on cognitive aging. Arterioscler Thromb Vasc Biol. 2021;41:1255–64.
Lee HC, Shiou YL, Jhuo SJ, Chang CY, Liu PL, Jhuang WJ, Dai ZK, Chen WY, Chen YF, Lee AS. The sodium-glucose co-transporter 2 inhibitor empagliflozin attenuates cardiac fibrosis and improves ventricular hemodynamics in hypertensive heart failure rats. Cardiovasc Diabetol. 2019;18:45.
Hu D, Dong R, Yang Y, Chen Z, Tang Y, Fu M, Wang DW, Xu X, Tu L. Human kallikrein overexpression alleviates cardiac aging by alternatively regulating macrophage polarization in aged rats. FASEB J. 2019;33:8436–52.
Daud E, Ertracht O, Bandel N, Moady G, Shehadeh M, Reuveni T, Atar S. The impact of empagliflozin on cardiac physiology and fibrosis early after myocardial infarction in non-diabetic rats. Cardiovasc Diabetol. 2021;20:132.
Weng L, Ye J, Yang F, Jia S, Leng M, Jia B, Xu C, Zhao Y, Liu R, Xiong Y, Zhou Y, Zhao J, Zheng M. TGF-β1/SMAD3 regulates programmed cell death 5 that suppresses cardiac fibrosis post-myocardial infarction by inhibiting HDAC3. Circ Res. 2023;133:237–51.
Lindner M, Böhle T, Beuckelmann DJ. Ca2+-handling in heart failure—a review focusing on Ca2+ sparks. Basic Res Cardiol. 2002;97(Suppl 1):I79–82.
Zelniker TA, Bonaca MP, Furtado R, Mosenzon O, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding J, Budaj A, Kiss RG, Padilla F, Gause-Nilsson I, Langkilde AM, Raz I, Sabatine MS, Wiviott SD. Effect of dapagliflozin on atrial fibrillation in patients with type 2 diabetes mellitus: insights from the DECLARE-TIMI 58 trial. Circulation. 2020;141:1227–34.
Lin YW, Chen CY, Shih JY, Cheng BC, Chang CP, Lin MT, Ho CH, Chen ZC, Fisch S, Chang WT. Dapagliflozin improves cardiac hemodynamics and mitigates arrhythmogenesis in mitral regurgitation-induced myocardial dysfunction. J Am Heart Assoc. 2021;10: e19274.
Shao Q, Meng L, Lee S, Tse G, Gong M, Zhang Z, Zhao J, Zhao Y, Li G, Liu T. Empagliflozin, a sodium glucose co-transporter-2 inhibitor, alleviates atrial remodeling and improves mitochondrial function in high-fat diet/streptozotocin-induced diabetic rats. Cardiovasc Diabetol. 2019;18:165.
Peters CH, Sharpe EJ, Proenza C. Cardiac pacemaker activity and aging. Annu Rev Physiol. 2020;82:21–43.
Jing Y, Yang R, Chen W, Ye Q. Anti-arrhythmic effects of sodium-glucose co-transporter 2 inhibitors. Front Pharmacol. 2022;13: 898718.
Senoner T, Dichtl W. Oxidative stress in cardiovascular diseases: still a therapeutic target? Nutrients. 2019;11:2090.
Durak A, Olgar Y, Degirmenci S, Akkus E, Tuncay E, Turan B. A SGLT2 inhibitor dapagliflozin suppresses prolonged ventricular-repolarization through augmentation of mitochondrial function in insulin-resistant metabolic syndrome rats. Cardiovasc Diabetol. 2018;17:144.
Mizuno M, Kuno A, Yano T, Miki T, Oshima H, Sato T, Nakata K, Kimura Y, Tanno M, Miura T. Empagliflozin normalizes the size and number of mitochondria and prevents reduction in mitochondrial size after myocardial infarction in diabetic hearts. Physiol Rep. 2018;6: e13741.
Pinto E. Blood pressure and ageing. Postgrad Med J. 2007;83:109–14.
Wright JJ, Fine LJ, Lackland DT, Ogedegbe G, Dennison HC. Evidence supporting a systolic blood pressure goal of less than 150 mmHg in patients aged 60 years or older: the minority view. Ann Intern Med. 2014;160:499–503.
Striepe K, Jumar A, Ott C, Karg MV, Schneider MP, Kannenkeril D, Schmieder RE. Effects of the selective sodium-glucose cotransporter 2 inhibitor empagliflozin on vascular function and central hemodynamics in patients with type 2 diabetes mellitus. Circulation. 2017;136:1167–9.
Bode B, Stenlöf K, Harris S, Sullivan D, Fung A, Usiskin K, Meininger G. Long-term efficacy and safety of canagliflozin over 104 weeks in patients aged 55–80 years with type 2 diabetes. Diabetes Obes Metab. 2015;17:294–303.
Sinclair A, Bode B, Harris S, Vijapurkar U, Mayer C, Fung A, Shaw W, Usiskin K, Desai M, Meininger G. Efficacy and safety of canagliflozin compared with placebo in older patients with type 2 diabetes mellitus: a pooled analysis of clinical studies. BMC Endocr Disord. 2014;14:37.
Bode B, Stenlöf K, Sullivan D, Fung A, Usiskin K. Efficacy and safety of canagliflozin treatment in older subjects with type 2 diabetes mellitus: a randomized trial. Hosp Pract. 1995;2013(41):72–84.
Chilton R, Tikkanen I, Cannon CP, Crowe S, Woerle HJ, Broedl UC, Johansen OE. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17:1180–93.
Maliha G, Townsend RR. SGLT2 inhibitors: their potential reduction in blood pressure. J Am Soc Hypertens. 2015;9:48–53.
Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85:962–71.
Lambers HH, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15:853–62.
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC, Fedson SE, Fonarow GC, Hayek SS, Hernandez AF, Khazanie P, Kittleson MM, Lee CS, Link MS, Milano CA, Nnacheta LC, Sandhu AT, Stevenson LW, Vardeny O, Vest AR, Yancy CW. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895–1032.
Luo F, Das A, Chen J, Wu P, Li X, Fang Z. Metformin in patients with and without diabetes: a paradigm shift in cardiovascular disease management. Cardiovasc Diabetol 2019;18(1):54.
Škrtić M, Cherney DZ. Sodium-glucose cotransporter-2 inhibition and the potential for renal protection in diabetic nephropathy. Curr Opin Nephrol Hypertens. 2015;24:96–103.
Acknowledgements
We thank Sarah Ivins, Ph.D., from Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the English text of a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Source of funding
This research was funded by grants from Sichuan Science and Technology Program (2022YFS0610), Luzhou Municipal People’s Government—Southwest Medical University Science and Technology Strategic Cooperation (2021LZXNYD-J33), Hejiang People’s Hospital—Southwest Medical University Science and Technology Strategic Cooperation Project (2021HJXNYD13), Gulin County People’s Hospital—Affiliated Hospital of Southwest Medical University Science and Technology strategic Cooperation (2022GLXNYDFY13) and 2022-N-01-33 project of China International Medical Foundation.
Conflict of interest
Yali Zhang, Yufeng He, Siqi Liu, Li Deng, Yumei Zuo, Keming Huang, Bin Liao, Guang Li, and Jian Feng declare that they have no conflict of interest that might be relevant to this manuscript.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Data availability statement
The data used to support the findings of this study are included within the article.
Code availability
Not applicable.
Author contributions
YZ, YH, and SL conceived, designed, and planned the manuscript. YZ, KH, and BL collected and read the literature. YZ drafted the manuscript. LD made extensive revisions to the manuscript during the revision process. JF and GL conceived, designed, and revised the manuscript. All authors read and approved the final manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., He, Y., Liu, S. et al. SGLT2 Inhibitors in Aging-Related Cardiovascular Disease: A Review of Potential Mechanisms. Am J Cardiovasc Drugs 23, 641–662 (2023). https://doi.org/10.1007/s40256-023-00602-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-023-00602-8