Abstract
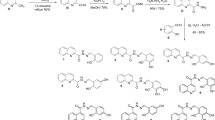
In this study, we used the improved extreme-difference normalization method to calculate the comprehensive evaluation values of bioenrichment and toxicity of benzophenone UV light absorbers(BPs). Based on this parameter, a 3D-QSAR(QSAR=quantitative structure activity relationship) pharmacophore model was constructed using Discovery Studio software and applied to the molecular modification of BPs. With three commonly used ingredients in sunscreen 2-hydroxy-4-methoxybenzophenone(BP-3), 2,2′-dihydroxy-4,4′-dimethoxybenzophenone(BP-6) and 2,2′-dihydroxy-4-methoxybenzophenone(BP-8) as target molecules, we performed BPs substitution reaction based on the binding positions of characteristic elements of the pharmacophore model and designed BP derivatives with reduced bioenrichment and toxicity. Stability and function evaluation showed that while the stability of 6 BP derivatives was enhanced, the light absorption capacity was also significantly enhanced(from 9.16% to 43.16%). Molecular dynamics simulation results showed that the binding ability of BP-609 molecule with serum albumin was reduced by 16.37% compared with BP-6, and the binding with collagen could not occur spontaneously, which could be used as an explanation for the simultaneous reduction of its bioenrichment and toxicity. Besides, through the simulation of human metabolism, it was found that the liver metabolites of BP-609 were less toxic, which reduced the potential risk of human metabolism. It proved that the molecular modification scheme of BPs was environment-friendly.
Similar content being viewed by others
References
Negreira N., Rodríguez I., Rodil R., Cela R., Anal. Chim. Acta, 2012, 743, 101
Kim S., Choi K., Environ. Int., 2014, 70, 143
Liao C. Y., Kannan K., Environ. Sci. Technol., 2014, 48(7), 4103
Zheng J., Daily Chemical Science, 2010, 33(8), 18
Balmer M. E., Buser H. R., Müller M. D., Poiger T., Plant Protecting Chemistry, 2005, 39, 953
Sun H. Q., The Ecotoxicological Study about the Personal Care Products in Slightly-Polluted Water, Southeast University, 2016
DíazCruz M. S., GagoFerrero P., Llorca M., Barcelo D., Anal. Bioanal. Chem., 2012, 402(7), 2325
Wang L., Asimakopoulos A. G., Moon H., Nakata H., Kannan K., Environ. Sci. Technol., 2013, 47(9), 4752
Peng X. Z., Jin J. B., Wang C. W., Ou W. H., Tang C. M., J. Chromatogr. A, 2015, 1384, 97
Rodríguez-Gómez R., Zafra-Gómez A., Camino-Sánchez F. J., Ballesteros O., Navalón A., J. Chromatogr. A, 2014, 1349, 69
Zhang T., Sun H. W., Qin X. L., Wu Q., Zhang, Y. F., Ma J., Kannan K., Sci. Total. Environ., 2013, 461, 49
Gao C. J., Liu L. Y., Ma W. L., Zhu N. Z., Jiang L., Li Y. F., Kannan K., Environ. Pollut., 2015, 203, 1
Wang L., Asimakopoulos A. G., Kannan K., Environ. Int., 2015, 78, 45
VelaSoria F., Rodríguez I., Ballesteros O., Zafragomez A., Ballesteros L., Cela R., Navalón A., J. Chromatogr. A, 2014, 1371, 39
Zucchi S., Blüthgen N., Ieronimo A., Fent K. Toxicol. Appl. Pharm., 2011, 250(2), 137
Blüthgen N., Zucchi S., Fent K., Toxicol. Appl. Pharm., 2012, 263(2), 184
Zeiger E., Anderson B., Haworth S., Lawlor T., Mortelmans K., Environ. Mol. Mutagen., 1992, 19, 2
Zhang F., Zhang J., Tong C. L., Chen Y. D., Zhuang S. L., Liu W. P., J. Hazard. Mater., 2013, 263, 618
Schlumpf M., Cotton B., Conscience M., Haller V., Steinmann B., Lichtensteiger W., Environ Health. Persp., 2001, 109, 239
Ministry of Health of the People’s Republic of China, GB 7916-87 Cosmetic Hygiene Standards, China Standard Press, Beijing, 1987
EEC(European Economic Community), Directive 83/574., 1983, L332, 38
FDA(U.S. Food and Drug Administration), Register Rules Regs., 1999, 64, 2766
MHLW(Ministry of Health and Welfare), Japanese Standards of Cosmetic Ingredients, MHLW Notification No. 331 of 2000, Tokyo, 2000
Jeon H. K., Chung Y., Ryu J. C., J. Chromatogr. A, 2006, 1131(1), 192
The International Chemical Secretariat(ChemSec) Version 2 SIN list includes 22 endocrine disruptive substances, Available at: http://www.cirsgroup.com/reg/news/reach/3549.html. (November 2020)
EPA Adds 23 Chemicals, including BPA, to key list for scrutiny, possible action, Available at: https://pubs.acs.org/doi/abs/10.1021/cen-09244-notw5. (November 2020)
Wang H., Xu X., Zhang D., Resources Economization & Environment Protection, 2018, 4, 82
Ren Z. X., Wang Y. W., Xu H. H., Li Y. F., Han S., Int. J. Env. Res. Pub. He., 2019, 16(17), 31
Li M. H., Toxicol. Environ. Chem., 2012, 94, 566
Xue F., Study on the interaction between vanillin and protein, Shanghai Normal University, 2016
Zhang F., Moleclar mechanism of the interaction of UV-filters with serum albumin, Zhejiang University, 2014
Pollesch N. L., Dale V. H., Ecol. Econ., 2016, 130, 195
Huang H. J., Lee C. C., Chen C. Y., Evid-Based Compl. Alt., 2014, 2014, 1
Sanderson H., Fauser P., Thomsen M., Sørensen P. B., J. Hazard. Mater., 2007, 154, 846
Tan Y., Han X. J., Liu D. S., Shen N., Shen S. G., Chinese Journal of Experimental Traditional Medical Formulae, 2016, 22(15), 199
Lin K. J., Huang Z. G., Li T., Wang N. X., Cheng Y., Shen L. L., W Y. T., Li H., Ye B. P., Chem. J. Chinese Universities, 2013, 34(5), 1240
Arooj M., Thangapandian S., John S., Hwang S., Park J. K., Lee K. W., Int. J. Mol. Sci., 2011, 12(12), 9236
Nayana R. S., Bommisetty S. K., Singh K., Bairy S. K., Nunna S., Pramod A., Muttineni R., J. Chem. Inf. Model., 2009, 49(1), 53
Qiu Y. L., Xin M. L., Li Y., Spectrosc. Spect. Anal., 2018, 38(2), 441
Frisch M. J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Mennucci B., Petersson G. A., Nakatsuji H., Caricato M., Li X., Hratchian H. P., Izmaylov A. F., Bloino J., Zheng G., Sonnenberg J. L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery Jr. J. A., Peralta J. E., Ogliaro F., Bearpark M., Heyd J. J., Brothers E., Kudin K. N., Staroverov V. N., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Rega N., Millam J. M., Klene M., Knox J. E., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Martin R. L., Morokuma K., Zakrzewski V. G., Voth G. A., Salvador P., Dannenberg J. J., Dapprich S., Daniels A. D., Farkas Ö., Foresman J. B., Ortiz J. V., Cioslowski J., Fox D. J., Gaussian 09, Gaussian, Inc., Wallingford CT, 2009
Qiu Y. L., Jiang L., Li Y., Spectrosc. Lett., 2018, 51, 155
Chen K. X., Jiang H. L., Ji R. Y., Computer aided drug design: principles, methods and applications, Shanghai Science and Technology Press, Shanghai, 2000
Cao Y. M., Xu L., Jia L. Y., New Biotechnol., 2011, 29(1), 90
Jaworska J. S., Comber M., Auer C., Leeuwen C. V., Environ. Health. Persp., 2003, 111, 1358
Zhao Y. Y., Gu W. W., Li Y., J. Mol. Graph. Model., 2018, 84, 197
Wang H. X., Qin D. Y., Yue Y. K., Bo W. C., Zheng X., Liang G. Z., Journal of Molecular Science, 2020, 36(3), 191
Shao X., Yi Q. Y., Yang C. L., Journal of China Pharmaceutical University, 2016, 47(1), 38
Jiang L., Li Y., J. Hazard. Mater., 2016, 307, 202
Gissi A., Lombardo A., Roncaglioni A., Gadaleta D., Mangiatordi G. F., Nicolotti O., Benfenati E., Environ. Res., 2015, 137, 398
Todorova L., Vassileva V., Fibres. Text. East. Eur., 2003, 11, 21
Hanson K. M., Gratton E., Bardeen C. J., Free. Radical. Bio. Med., 2006, 41(8), 1205
Wang L. H., Wang W. H., Guo Y. X., Yang J., Heilongjiang Agricultural Science, 2014, 3, 150
Xu G. J., A QSAR Study on the Quantitative Structure and Toxicity of Phenol and Aniline Compounds, Zhejiang University, 2003
Watanabe Y., Kojima H., Takeuchi S., Uramaru N., Sanoh S., Sugihara K., Kitamura S., Ohta S., Toxicol. Appl. Pharm., 2015, 282(2), 119
Hosseini F., Ariafard A., Rashidi M., Azimi G., Nabavizadeh S. M., J. Organomet. Chem., 2011, 696(21), 3351
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Xiao, J., Zhang, W., Zhang, S. et al. Molecular Modification of Benzophenone Derivatives for Lower Bioenrichment and Toxicity Through the Pharmacophore Model. Chem. Res. Chin. Univ. 38, 535–545 (2022). https://doi.org/10.1007/s40242-021-1044-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40242-021-1044-3