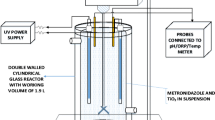
Abstract
In this study, the removal efficiency of endosulfan as a persistent organic pollutant and formation of its metabolites were investigated using Ag/TiO2/Fe3O4 photocatalyst under visible and UV-A light. Light intensity, catalyst amount, initial endosulfan concentration, initial pH and time were determined as controllable factors for Taguchi experimental design. The highest removal efficiencies of endosulfan were achieved as 86.14% and 85.46% for visible and UV–A light sources, respectively. According to the greatest best criterion, the level at which the highest S/N ratio was obtained for each parameter was accepted as the optimum value. As a result of the validation experiments, 94.2% and 91.9% efficiency were obtained for visible and UV-A light, respectively. The metabolite formations of endosulfan (endosulfan sulfate, ether, and lactone) remained below 7% in all experiments on a concentration basis. In the reuse experiments of the magnetically recovered photocatalyst, high removal efficiency of around 80% was obtained after four cycles. The removal efficiencies were found to be 86.7% and 84.8%, for real samples taken from the drinking water treatment plant inlet and the spring water network injected with endosulfan under optimal photocatalysis experimental conditions, respectively. It has been shown that nitrate and sulfate anions, which are in significant concentrations in raw water samples, have very little effects on endosulfan removal. The overall results showed that the Ag/TiO2/Fe3O4 photocatalyst was produced successfully, the catalyst was highly effective in the mineralization of endosulfan in synthetic and real water samples under UV and visible light, and effective yields could be obtained even with reuse.
Highlights
-
Endosulfan removal efficiencies of 94.2%, 86.7% and 84.8% were achieved in synthetic, raw water and spring water samples, respectively, and no formation any metabolites under visible light.
-
77.23% removal efficiency was obtained even after five consecutive uses of the catalyst.
-
A major advantage of this technology is that no chemical oxidants are added in the process and no sludge is produced as a reaction residue.
-
Taguchi and ANOVA analyzes showed that the most effective parameters in endosulfan removal were initial endosulfan dose and pH.









Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Li Y, Macdonald R. Sources and pathways of selected organochlorine pesticides to the Arctic and the effect of pathway divergence on HCH trends in biota: a review. Sci Total Environ. 2005;342(1–3):87–106.
Weber J, et al. Endosulfan, a global pesticide: a review of its fate in the environment and occurrence in the Arctic. Sci Total Environ. 2010;408(15):2966–84.
Ray AK. A new photocatalytic reactor for destruction of toxic water pollutants by advanced oxidation process. Catal Today. 1998;44(1–4):357–68.
Shahrezaei F, et al. Process modeling and kinetic evaluation of petroleum refinery wastewater treatment in a photocatalytic reactor using TiO2 nanoparticles. Powder Technol. 2012;221:203–12.
Chowdhury ZZ, et al. A review on electrochemically modified carbon nanotubes (CNTs) membrane for desalination and purification of water. Mater Res Express. 2018;5(10):102001.
Nasirian M, Bustillo-Lecompte CF, Mehrvar M. Photocatalytic efficiency of Fe2O3/TiO2 for the degradation of typical dyes in textile industries: effects of calcination temperature and UV-assisted thermal synthesis. J Environ Manag. 2017;196:487–98.
Liu C, et al. The removal of DON derived from algae cells by Cu-doped TiO2 under sunlight irradiation. Chem Eng J. 2015;280:588–96.
Sarteep Z, Ebrahimian Pirbazari A, Aroon MA. Silver doped TiO2 nanoparticles: preparation, characterization and efficient degradation of 2, 4-dichlorophenol under visible light. J Water Environ Nanatechnol. 2016;1(2):135–44.
Chirita M, et al. Fe2O3–nanoparticles, physical properties and their photochemical and photoelectrochemical applications. Chem Bull. 2009;54(68):1–8.
Laurent S, et al. Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev. 2008;108(6):2064–110.
Xu L, et al. Direct evidence for the interfacial oxidation of CO with hydroxyls catalyzed by Pt/oxide nanocatalysts. J Am Chem Soc. 2009;131(45):16366–7.
Cai Z, et al. An overview of nanomaterials applied for removing dyes from wastewater. Environ Sci Pollut Res. 2017;24(19):15882–904.
Mishra M, Chun D-M. α-Fe2O3 as a photocatalytic material: a review. Appl Catal A. 2015;498:126–41.
Turkyilmaz M, Kucukcongar S. Investigation of Endosulfan removal and metabolite formation by Photocatalysis process under UV-C light source using Taguchi Experimental Design Program. Int J Environ Anal Chem. 2022;1–14.
Fisli A, Saridewi R, Dewi SH, Gunlazuardi J. Preparation and characterization of Fe3O4/TiO2 composites by heteroagglomeration. In Advanced Materials Research. Trans Tech Publ. 2013;626:131–137.
Dewi SH, Fisli A, Wardiyati S. Synthesis and characterization of magnetized photocatalyst Fe3O4/SiO2/TiO2 by heteroagglomeration method. in Journal of Physics: Conference Series. 2016. IOP Publishing.
Alwindawi AGJ, Turkyilmaz M, Kucukcongar S. Synthesis of magnetic photocatalyst by photochemical deposition and co-precipitation techniques: investigation of its photocatalytic and sonophotocatalytic activity for dye removal. Int J Environ Anal Chem. 2022;102(19):8419–33.
Ko S, Banerjee CK, Sankar J. Photochemical synthesis and photocatalytic activity in simulated solar light of nanosized Ag doped TiO2 nanoparticle composite. Compos Part B Eng. 2011;42(3):579–83.
Yiantzi E, et al. Vortex-assisted liquid–liquid microextraction of octylphenol, nonylphenol and bisphenol-A. Talanta. 2010;80(5):2057–62.
Türkyılmaz M, Küçükçongar S. Endosülfan ve Metabolitlerinin Su Örneklerinde Vorteks Destekli Sıvı-Sıvı Mikro Ekstraksiyon ve Yüksek Performanslı Sıvı Kromatografi Kullanılarak Analizi. Bitlis Eren Üniversitesi Fen Bilimleri Dergisi. 2021;10(4):1404–15.
Taguchi G, Chowdhury S, Wu Y. Taguchi’s quality engineering handbook. Wiley; 2005.
TTaguchi G, Yuin Wo. Taguchi methods: case studies from the US and Europe. Amer Supplier Inst. 1989;6.
Sowmya S, Madhu G, Hashir M. Studies on nano-engineered TiO2 photo catalyst for effective degradation of dye. in IOP Conference Series: Materials Science and Engineering. 2018. IOP Publishing.
Tang J, et al. Construction of novel Z-scheme Ag/FeTiO3/Ag/BiFeO3 photocatalyst with enhanced visible-light-driven photocatalytic performance for degradation of norfloxacin. Chem Eng J. 2018;351:1056–66.
Zhang L, et al. Preparation of magnetic Fe3O4/TiO2/Ag composite microspheres with enhanced photocatalytic activity. Solid State Sci. 2016;52:42–8.
Zhan J, Zhang H, Zhu G. Magnetic photocatalysts of cenospheres coated with Fe3O4/TiO2 core/shell nanoparticles decorated with ag nanopartilces. Ceram Int. 2014;40(6):8547–59.
Tachikawa T, Fujitsuka M, Majima T. Mechanistic insight into the TiO2 photocatalytic reactions: design of new photocatalysts. J Phys Chem C. 2007;111(14):5259–75.
Guo J, et al. Adsorption and photocatalytic degradation behaviors of rhodamine dyes on surface-fluorinated TiO 2 under visible irradiation. RSC Adv. 2016;6(5):4090–100.
Gusain R, et al. Adsorptive removal and photocatalytic degradation of organic pollutants using metal oxides and their composites: a comprehensive review. Adv Colloid Interface Sci. 2019;272: 102009.
Ismael A, et al. Novel TiO 2/GO/CuFe 2 O 4 nanocomposite: a magnetic, reusable and visible-light-driven photocatalyst for efficient photocatalytic removal of chlorinated pesticides from wastewater. RSC Adv. 2020;10(57):34806–14.
Das K, et al. Facile synthesis and application of CdS/Bi20TiO32/Bi4Ti3O12 ternary heterostructure: a synergistic multi-heterojunction photocatalyst for enhanced endosulfan degradation and hydrogen evolution reaction. Appl Catal B. 2022;303: 120902.
Nekooie R, et al. Design and synthesis of g-C3N4/(Cu/TiO2) nanocomposite for the visible light photocatalytic degradation of endosulfan in aqueous solutions. J Mol Struct. 2022;1258: 132650.
Goel P, Arora M. Photocatalytic degradation efficiency of Cu/Cu2O core–shell structured nanoparticles for endosulfan mineralization. J Nanopart Res. 2022;24(3):1–13.
Zangeneh H, et al. Photocatalytic oxidation of organic dyes and pollutants in wastewater using different modified titanium dioxides: a comparative review. J Ind Eng Chem. 2015;26:1–36.
Maki LK, et al. LED-activated immobilized Fe-Ce-N tri-doped TiO2 nanocatalyst on glass bed for photocatalytic degradation organic dye from aqueous solutions. Volume 15. Environ Technol Innov. 2019. p. 100411.
Cheng HY, Chang KC, Lin KL, Ma CM. Study on isopropanol degradation by UV/TiO2 nanotube. In: AIP Conference Proceedings, vol 1946, no 1. AIP Publishing LLC. 2018; p. 020006.
Daneshvar N, Salari D, Khataee A. Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2. J Photochem Photobiol A. 2004;162(2–3):317–22.
Herrmann J-M. Heterogeneous photocatalysis: fundamentals and applications to the removal of various types of aqueous pollutants. Catal Today. 1999;53(1):115–29.
Mohabansi N, Patil V, Yenkie N. A comparative study on photo degradation of methylene blue dye effluent by advanced oxidation process by using TiO2/ZnO photo catalyst. Rasayan J Chem. 2011;4(4):814–9.
Wu R-J, et al. Phorate degradation by TiO2 photocatalysis: parameter and reaction pathway investigations. Desalination. 2010;250(3):869–75.
Konstantinou IK, Albanis TA. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations: a review. Appl Catal B. 2004;49(1):1–14.
Huang M, et al. Photocatalytic discolorization of methyl orange solution by pt modified TiO2 loaded on natural zeolite. Dyes Pigm. 2008;77(2):327–34.
Lu D, et al. Enhanced photocatalytic degradation of aqueous phenol and cr (VI) over visible-light-driven TbxOy loaded TiO2-oriented nanosheets. Appl Surf Sci. 2017;399:167–84.
Abbas T, et al. Iron turning waste media for treating Endosulfan and Heptachlor contaminated water. Sci Total Environ. 2019;685:124–33.
Dong J, et al. Effects of pH and particle size on kinetics of nitrobenzene reduction by zero-valent iron. J Environ Sci. 2010;22(11):1741–7.
Yin W, et al. Experimental study of zero-valent iron induced nitrobenzene reduction in groundwater: the effects of pH, iron dosage, oxygen and common dissolved anions. Chem Eng J. 2012;184:198–204.
Shih Y-h, Tai Y-t. Reaction of decabrominated diphenyl ether by zerovalent iron nanoparticles. Chemosphere. 2010;78(10):1200–6.
Mozia S. Photocatalytic membrane reactors (PMRs) in water and wastewater treatment. A review. Sep Purif Technol. 2010;73(2):71–91.
Çalışkan Y, Yatmaz HC, Bektaş N. Photocatalytic oxidation of high concentrated dye solutions enhanced by hydrodynamic cavitation in a pilot reactor. Process Saf Environ Prot. 2017;111:428–38.
McCullagh C, et al. Development of a slurry continuous flow reactor for photocatalytic treatment of industrial waste water. J Photochem Photobiol A. 2010;211(1):42–6.
Sivagami K, et al. Chlorpyrifos and Endosulfan degradation studies in an annular slurry photo reactor. Ecotoxicol Environ Saf. 2016;134:327–31.
Shah NS, et al. Comparative studies of various iron-mediated oxidative systems for the photochemical degradation of endosulfan in aqueous solution. J Photochem Photobiol A. 2015;306:80–6.
Özçelep B. Kağıt endüstrisi atıksularının membran prosesleriyle ileri arıtımı. İstanbul: Doktora Tezi, İstanbul Teknik Üniversitesi, Fen Bilimleri Enstitüsü; 2009.
Land CE. Confidence intervals for linear functions of the normal mean and variance. Ann Math Stat. 1971;1187–1205.
Shah NS, et al. Efficient removal of endosulfan from aqueous solution by UV-C/peroxides: a comparative study. J Hazard Mater. 2013;263:584–92.
Hayon E, Treinin A, Wilf J. Electronic spectra, photochemistry, and autoxidation mechanism of the sulfite-bisulfite-pyrosulfite systems. SO2-, SO3-, SO4-, and SO5-radicals. J Am Chem Soc. 1972;94(1):47–57.
Liang C, Wang Z-S, Mohanty N. Influences of carbonate and chloride ions on persulfate oxidation of trichloroethylene at 20 C. Sci Total Environ. 2006;370(2–3):271–7.
Bennedsen LR, Muff J, Søgaard EG. Influence of chloride and carbonates on the reactivity of activated persulfate. Chemosphere. 2012;86(11):1092–7.
Grebel JE, Pignatello JJ, Mitch WA. Effect of halide ions and carbonates on organic contaminant degradation by hydroxyl radical-based advanced oxidation processes in saline waters. Environ Sci Technol. 2010;44:6822 (8. 17).
Acknowledgements
I would like to thank the Republic of Turkey Higher Education Institution for the financial support it has provided with the 2014-ÖYP-106 project.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Mehmet Turkyilmaz and Sezen Kucukcongar. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
ESM 1
Characterization values of raw water and spring water samples taken from Konya Water and Sewerage Administration are given in supplementary data 1. (PDF 1.62 mb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Turkyilmaz, M., Kucukcongar, S. A comparison of endosulfan removal by photocatalysis process under UV-A and visible light irradiation: optimization, degradation byproducts and reuse. J Environ Health Sci Engineer 21, 355–371 (2023). https://doi.org/10.1007/s40201-023-00864-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40201-023-00864-z