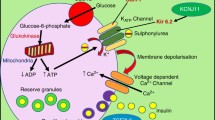
Abstract
Purpose
Although metformin is the first-line treatment of type 2 diabetes mellitus (T2DM), a few studies have evaluated the benefits of monotherapies (metformin) versus combination therapy (metformin and glibenclamide) for treatment of T2DM patients. The present study aimed to evaluate the effect of monotherapy with metformin compared to combination therapy with metformin and glibenclamide on the expression of RAGE, Nrf 2, and Sirt1genes.
Methods
EightyT2DM patients and 40 healthy individuals participated in this case-control study. The patients in the treatment group were divided into two groups who received either metformin alone (n = 40) or metformin in combination with glibenclamide (n = 40). FBS, HbA1c, and fructosamine were measured. The expression of RAGE, Nrf 2, and Sirt 1 genes in PBMC of all subjects were assessed using real-time PCR.
Results
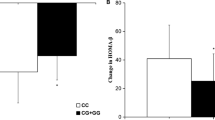
RAGE gene expression in both treatment groups was significantly lower than the control (P < 0.05). RAGE gene expression was significantly reduced in the combination of metformin and glibenclamide treated group compared to metformin group (P < 0.05). Additionally, the expression of Sirt 1 and Nrf 2 genes in both treatment groups was higher than that of the control group (P < 0.05). The expression of Sirt 1 and Nrf 2 genes in metformin and glibenclamide treated group were higher than the metformin group (P < 0.05).
Conclusion
Combination therapy (metformin and glibenclamide) showed stronger effect on repression of the RAGE gene and activation of Nrf 2 and Sirt 1 genes compared to monotherapy (metformin); therefore, it can be concluded that combination therapy may have more protective effects on the T2DM patients. No significant correlation was observed between HbA1c and RAGE, Sirt 1, and Nrf 2 genes expression.






Similar content being viewed by others
References
German M. Chapter 17. Pancreatic hormones and diabetes mellitus. Dalam: Gardner DG, Shoback D, eds. Greenspan’s basic & clinical endocrinology. New York: McGraw-Hill; 2011.
Sebastián C, Satterstrom FK, Haigis MC, Mostoslavsky R. From sirtuin biology to human diseases: an update. J Biol Chem. 2012;287(51):42444–52. https://doi.org/10.1074/jbc.R112.402768.PMC3522245.
Rhodes CJ. Type 2 diabetes-a matter of ß-cell life and death? Sci. 2005;307(5708):380–4.
Larsen R, Kronenberg H, et al. Williams textbook of endocrinology. 12th ed. Amsterdam: Elsevier Saunders; 2011.
Herder C, Roden M. Genetics of type 2 diabetes: pathophysiologic and clinical relevance. Eur J Clin Investig. 2011;41(6):679–92.
Rich SS. Still a geneticist's nightmare. Nature. 2016;536(7614):37–8.
Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–20.
Cohen MP, Shea E, Chen S, Shearman CW. Glycated albumin increases oxidative stress, activates NF-κB and extracellular signal-regulated kinase (ERK), and stimulates ERK-dependent transforming growth factor-β1 production in macrophage RAW cells. J Lab Clin Med. 2003;141(4):242–9.
Triñanes J, Salido E, Fernández J, Rufino M, González-Posada JM, Torres A, et al. Type 1 diabetes increases the expression of proinflammatory cytokines and adhesion molecules in the artery wall of candidate patients for kidney transplantation. Diabetes Care. 2012;35(2):427–33.
Schmidt AM, Stern D. Atherosclerosis and diabetes: the RAGE connection. Curr Atheroscler Rep. 2000;2(5):430–6.
Yamagishi S-i, Imaizumi T. Diabetic vascular complications: pathophysiology, biochemical basis and potential therapeutic strategy. Curr Pharm Des. 2005;11(18):2279–99.
Yamamoto T, Suzuki T, Kobayashi A, Wakabayashi J, Maher J, Motohashi H, et al. Physiological significance of reactive cysteine residues of Keap1 in determining Nrf2 activity. Mol Cell Biol. 28(8):2758–70.
Wu P, Yan Y, Ma L-l, Hou B-y, He Y-y, Zhang L, et al. Effects of the Nrf2 protein modulator salvianolic acid a alone or combined with metformin on diabetes-associated macrovascular and renal injury. J Biol Chem. 2016;291(42):22288–301.
Cheng J-T, Huang C-C, Liu I-M, Tzeng T-F, Chang CJ. Novel mechanism for plasma glucose–lowering action of metformin in streptozotocin-induced diabetic rats. Diabetes. 2006;55(3):819–25.
Kitada M, Kume S, Kanasaki K, Takeda-Watanabe A, Koya D. Sirtuins as possible drug targets in type 2 diabetes. Curr Drug Targets. 2013;14(6):622–36.
Ripsin CM, Kang H, Urban RJ. Management of blood glucose in type 2 diabetes mellitus. Am Fam Physician. 2009;79(1):29–36.
Palmer SC, Mavridis D, Nicolucci A, Johnson DW, Tonelli M, Craig JC, et al. Comparison of clinical outcomes and adverse events associated with glucose-lowering drugs in patients with type 2 diabetes: a meta-analysis. JAMA. 2016;316(3):313–24.
Tabish SA. Is diabetes becoming the biggest epidemic of the twenty-first century? Int J Health Sci. 2007;(2):1, V–VIII.
Caton PW, Nayuni NK, Kieswich J, Khan NQ, Yaqoob MM, Corder R. Metformin suppresses hepatic gluconeogenesis through induction of SIRT1 and GCN5. J Endocrinol. 2010;205(1):97.
Yan SF, Ramasamy R, Schmidt AM. Mechanisms of disease: advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat Rev Endocrinol. 2008;4(5):285–93.
Herold K, Moser B, Chen Y, Zeng S, Yan SF, Ramasamy R, et al. Receptor for advanced glycation end products (RAGE) in a dash to the rescue: inflammatory signals gone awry in the primal response to stress. J Leukoc Biol. 2007;82(2):204–12.
Ramasamy R, Vannucci SJ, Yan SSD, Herold K, Yan SF, Schmidt AM. Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiol. 2005;15(7):16R–28R.
Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058–70.
Williams B, Gallacher B, Patel H, Orme C. Glucose-induced protein kinase C activation regulates vascular permeability factor mRNA expression and peptide production by human vascular smooth muscle cells in vitro. Diabetes. 1997;46(9):1497–503.
Ishii H, Jirousek MR, Koya D, Takagi C, Xia P, Clermont A, et al. Amelioration of vascular dysfunctions in diabetic rats by an oral PKC β inhibitor. Sci. 1996;272(5262):728–31.
Cuadrado A, Manda G, Hassan A, Alcaraz MJ, Barbas C, Daiber A, et al. Transcription factor NRF2 as a therapeutic target for chronic diseases: a systems medicine approach. Pharmacol Rev. 2018;70(2):348–83.
Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem. 2008;283(29):20015–26.
Gadducci A, Biglia N, Tana R, Cosio S, Gallo M. Metformin use and gynecological cancers: a novel treatment option emerging from drug repositioning. Crit Rev Oncol/Hematol. 2016;105:73–83.
Mafauzy M. Repaglinide versus glibenclamide treatment of type 2 diabetes during Ramadan fasting. Diabetes Res Clin Pract. 2002;58(1):45–53.
Tosi F, Muggeo M, Brun E, Spiazzi G, Perobelli L, Zanolin E, et al. Combination treatment with metformin and glibenclamide versus single-drug therapies in type 2 diabetes mellitus: a randomized, double-blind, comparative study. Metabolism. 2003;52(7):862–7.
Ishibashi Y, Matsui T, Takeuchi M, Yamagishi S-i. Beneficial effects of metformin and irbesartan on advanced glycation end products (AGEs)–RAGE-induced proximal tubular cell injury. Pharmacol Res. 2012;65(3):297–302.
Chen X-F, Zou J-J, Tang W, Lin W-D, Sun L-L, Bao Y, et al. Metformin corrects RAGE overexpression caused signaling dysregulation and NF-κB targeted gene change. Int J Clin Exp Med. 2016;9(2):2913–20.
Ishibashi Y, Matsui T, Takeuchi M, Yamagishi S. Metformin inhibits advanced glycation end products (AGEs)-induced renal tubular cell injury by suppressing reactive oxygen species generation via reducing receptor for AGEs (RAGE) expression. Horm Metab Res. 2012;44(12):891–5.
Gu J, Ye S, Wang S, Sun W, Hu Y. Metformin inhibits nuclear factor-κB activation and inflammatory cytokines expression induced by high glucose via adenosine monophosphate-activated protein kinase activation in rat glomerular mesangial cellsin vitro. Chin Med J. 2014;127(9):1755–60.
Hattori Y, Suzuki K, Hattori S, Kasai K. Metformin inhibits cytokine-induced nuclear factor κB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension. 2006;47(6):1183–8.
Ayers D, Baron B, Hunter T. miRNA influences in NRF2 pathway interactions within cancer models. J Nucleic Acids. 2015;2015:143636.
Leibiger IB, Berggren P-O. Sirt1: a metabolic master switch that modulates lifespan. Nat Med. 2006;12(1):34–6.
Hundal RS, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49(12):2063–9.
Acknowledgements
The present article was supported by the Transplant Research Center, Shiraz University of Medical Sciences, Shiraz, Iran. The study team would like to acknowledge gratefully the staff of this center.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding authors states that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hosseinipoor, H., Kariminejad, S.Y., Salehi, M. et al. The effects of metformin monotherapy and combination of metformin and glibenclamide therapy on the expression of RAGE, Sirt1, and Nrf2 genes in peripheral blood mononuclear cells of type 2 diabetic patients. J Diabetes Metab Disord 21, 369–377 (2022). https://doi.org/10.1007/s40200-022-00984-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-022-00984-7