Abstract
Background
Sildenafil is used to treat erectile dysfunction and pulmonary arterial hypertension and is metabolized in the liver mainly by CYP3A4, thus co-administration with drugs or herbal extracts that affect CYP3A4 activity may lead to drug-drug or drug-herb interactions, respectively. The aim of the present study was to evaluate the influence of single and multiple oral doses of methylxanthine fraction, isolated from Bancha green tea leaves on the pharmacokinetics of sildenafil in rats.
Methods
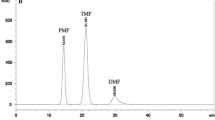
Rats were given sildenafil alone as well as simultaneously with methylxanthines or ketoconazole. The plasma concentrations of sildenafil were measured with high-performance liquid chromatography method with ultraviolet detection. The pharmacokinetic parameters of sildenafil were calculated by non-compartmental analysis.
Results
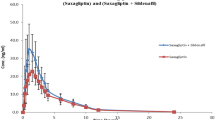
Concomitant use of sildenafil with a single oral dose of methylxanthines resulted in a decrease in Cmax (p > 0.05), AUC0-t (p < 0.05) and AUC0-inf (p < 0.05), while the administration of sildenafil after methylxanthines pretreatment resulted in an increase in Cmax (p < 0.0001), AUC0-t (p < 0.0001) and AUC0-inf (p < 0.001) compared to the sildenafil group. After co-administration of sildenafil and ketoconazole, a significant increase in Cmax, AUC0-t and AUC0-inf was observed in both of the experiments.
Conclusion
Drug-herb interactions were observed when sildenafil was co-administered with Bancha methylxanthines in rats. Further in vivo studies about the potential drug interactions between sildenafil and methylxanthines, especially caffeine, are needed to clarify mechanisms underlying the observed changes in sildenafil pharmacokinetics.
Graphical abstract








Similar content being viewed by others
Data availability
All data are included within the article.
References
Prasanth MI, Sivamaruthi BS, Chaiyasut C, Tencomnao T. A review of the role of green tea (Camellia sinensis) in Antiphotoaging, stress resistance, neuroprotection, and autophagy. Nutrients. 2019;11(2):474. https://doi.org/10.3390/nu11020474.
Tang G-Y, Meng X, Gan R-Y, Zhao C-N, Liu Q, Feng Y-B, et al. Health functions and related molecular mechanisms of tea components: an update review. Int J Mol Sci. 2019;20(24):6196. https://doi.org/10.3390/ijms20246196.
Gottwalt B, Tadi P. Methylxanthines. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK559165/: StatPearls Publishing LLC, 2021. Accessed 2021 15 Aug 2021
Monteiro JP, Alves MG, Oliveira PF, Silva BM. Structure-bioactivity relationships of Methylxanthines: trying to make sense of all the promises and the drawbacks. Molecules. 2016;21(8):974. https://doi.org/10.3390/molecules21080974.
Camandola S, Plick N, Mattson MP. Impact of coffee and cacao purine metabolites on neuroplasticity and neurodegenerative disease. Neurochem Res. 2019;44(1):214–27. https://doi.org/10.1007/s11064-018-2492-0.
Carrillo JA, Benitez J. Clinically significant pharmacokinetic interactions between dietary caffeine and medications. Clin Pharmacokinet. 2000;39(2):127–53. https://doi.org/10.2165/00003088-200039020-00004.
Briguglio M, Hrelia S, Malaguti M, Serpe L, Canaparo R, Dell'Osso B, et al. Food bioactive compounds and their interference in drug pharmacokinetic/Pharmacodynamic profiles. Pharmaceutics. 2018;10(4):277. https://doi.org/10.3390/pharmaceutics10040277.
Thorn CF, Aklillu E, McDonagh EM, Klein TE, Altman RB. PharmGKB summary: caffeine pathway. Pharmacogenet Genomics. 2012;22(5):389–95. https://doi.org/10.1097/FPC.0b013e3283505d5e.
Candela L, Formato M, Crescente G, Piccolella S, Pacifico S. Coumaroyl Flavonol glycosides and more in marketed green teas: an intrinsic value beyond much-lauded Catechins. Molecules. 2020;25(8):1765. https://doi.org/10.3390/molecules25081765.
Musial C, Kuban-Jankowska A, Gorska-Ponikowska M. Beneficial properties of green tea Catechins. Int J Mol Sci. 2020;21(5):1744. https://doi.org/10.3390/ijms21051744.
Iwasaki M, Inoue M, Sasazuki S, Sawada N, Yamaji T, Shimazu T, et al. Green tea drinking and subsequent risk of breast cancer in a population-based cohort of Japanese women. Breast Cancer Res. 2010;12(5):R88. https://doi.org/10.1186/bcr2756.
Makiuchi T, Sobue T, Kitamura T, Ishihara J, Sawada N, Iwasaki M, et al. Association between green tea/coffee consumption and biliary tract cancer: a population-based cohort study in Japan. Cancer Sci. 2016;107(1):76–83. https://doi.org/10.1111/cas.12843.
Burana-osot J, Yanpaisan W. Catechins and caffeine contents of green tea commercialized in Thailand. J Pharm Biomed Sci. 2012;22(22):17.
Andersson KE. PDE5 inhibitors - pharmacology and clinical applications 20 years after sildenafil discovery. Br J Pharmacol. 2018;175(13):2554–65. https://doi.org/10.1111/bph.14205.
Al-Mohizea AM, Ahad A, El-Maghraby GM, Al-Jenoobi FI, AlKharfy KM, Al-Suwayeh SA. Effects of Nigella sativa, Lepidium sativum and Trigonella foenum-graecum on sildenafil disposition in beagle dogs. Eur J Drug Metab Pharmacokinet. 2015;40(2):219–24. https://doi.org/10.1007/s13318-014-0199-4.
Abdelkawy KS, Donia AM, Turner RB, Elbarbry F. Effects of lemon and Seville Orange juices on the pharmacokinetic properties of sildenafil in healthy subjects. Drugs R D. 2016;16(3):271–8. https://doi.org/10.1007/s40268-016-0140-1.
Graziano S, Montana A, Zaami S, Rotolo M, Minutillo A, Busardò F, et al. Sildenafil-associated hepatoxicity: a review of the literature. Eur Rev Med Pharmacol Sci. 2017;21:17–22.
Hsueh TY, Wu Y-T, Lin L-C, Chiu AW, Lin C-H, Tsai T-H. Herb-drug interaction of Epimedium sagittatum (Sieb. Et Zucc.) maxim extract on the pharmacokinetics of sildenafil in rats. Molecules. 2013;18(6):7323–35. https://doi.org/10.3390/molecules18067323.
Mallah E, Walid S, Rayyan W, Abu Dayyih W, Elhajji F, Mansoor K, et al. Dose-dependent synergistic effect of pomegranate juice on the bioavailability of sildenafil in rats by using HPLC method. Lat Am J Pharm. 2016;35:1277–84.
Mallah E, Saleh S, Rayyan W, Abu Dayyih W, Elhajji F, Mima M, et al. The influence of Eruca sativa (arugula) on pharmacokinetics of sildenafil in rats. Neuro Endocrinol Lett. 2017;38:295–300.
Mekjaruskul C, Sripanidkulchai B. Pharmacokinetic interaction between Kaempferia parviflora extract and sildenafil in rats. J Nat Med. 2015;69(2):224–31. https://doi.org/10.1007/s11418-014-0882-4.
Georgiev KD, Radeva-Ilieva M, Stoeva S, Zhelev I. Isolation, analysis and in vitro assessment of CYP3A4 inhibition by methylxanthines extracted from Pu-erh and Bancha tea leaves. Sci Rep. 2019;9(1):13941. https://doi.org/10.1038/s41598-019-50468-7.
ICH. Topic Q2 (R1) Validation of analytical procedures: Text and methodology (CPMP/ ICH/ 381/ 95), 1995. 2005.
Mauvais-Jarvis F, Arnold AP, Reue K. A guide for the Design of pre-clinical Studies on sex differences in metabolism. Cell Metab. 2017;25(6):1216–30. https://doi.org/10.1016/j.cmet.2017.04.033.
Food and Drug Administration (FDA). Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers [database on the Internet]July 2005. Accessed: https://www.fda.gov/media/72309/download
Heimann M, Käsermann HP, Pfister R, Roth DR, Bürki K. Blood collection from the sublingual vein in mice and hamsters: a suitable alternative to retrobulbar technique that provides large volumes and minimizes tissue damage. Lab Anim. 2009;43(3):255–60. https://doi.org/10.1258/la.2008.007073.
Zhang Y, Huo M, Zhou J, Xie S. PKSolver: an add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft excel. Comput Methods Prog Biomed. 2010;99(3):306–14. https://doi.org/10.1016/j.cmpb.2010.01.007.
Mouly S, Lloret-Linares C, Sellier P-O, Sene D, Bergmann JF. Is the clinical relevance of drug-food and drug-herb interactions limited to grapefruit juice and saint-John’s wort? Pharmacol Res. 2017;118:82–92. https://doi.org/10.1016/j.phrs.2016.09.038.
Belayneh A, Molla F. The effect of coffee on pharmacokinetic properties of drugs : a review. Biomed Res Int. 2020;2020:7909703. https://doi.org/10.1155/2020/7909703.
Teschke R, Xuan TD. How can green tea polyphenols affect drug metabolism and should we be concerned? Expert Opin Drug Metab Toxicol. 2019;15(12):989–91. https://doi.org/10.1080/17425255.2019.1697228.
Sanchez JM. Methylxanthine content in commonly consumed foods in Spain and determination of its intake during consumption. Foods (Basel, Switzerland). 2017;6(12):109. https://doi.org/10.3390/foods6120109.
Hodges RE, Minich DM. Modulation of metabolic detoxification pathways using foods and food-derived components: a scientific review with clinical application. J Nutr Metab. 2015;2015:760689. https://doi.org/10.1155/2015/760689.
Dresser GK, Urquhart BL, Proniuk J, Tieu A, Freeman DJ, Arnold JM, et al. Coffee inhibition of CYP3A4 in vitro was not translated to a grapefruit-like pharmacokinetic interaction clinically. Pharmacol Res Perspect. 2017;5(5):e00346. https://doi.org/10.1002/prp2.346.
Mandlekar SV, Rose AV, Cornelius G, Sleczka B, Caporuscio C, Wang J, et al. Development of an in vivo rat screen model to predict pharmacokinetic interactions of CYP3A4 substrates. Xenobiotica. 2007;37(9):923–42. https://doi.org/10.1080/00498250701570269.
Schwartz B, Kloner R. Drug interactions with Phosphodiesterase-5 inhibitors used for the treatment of erectile dysfunction or pulmonary hypertension. Circulation. 2010;122:88–95. https://doi.org/10.1161/CIRCULATIONAHA.110.944603.
Jo JH, Kim SJ, Nam WS, Seung EJ, Lee S. Decreased absorption of midazolam in the stomach due to low pH induced by co-administration of Banha-sasim-tang. Environ Health Toxicol. 2016;31:e2016016. https://doi.org/10.5620/eht.e2016016.
Akula P, P.K L. Effect of pH on weakly acidic and basic model drugs and determination of their ex vivo transdermal permeation routes. Brazilian. J Pharm Sci. 2018;54:e00070. https://doi.org/10.1590/s2175-97902018000200070.
Vaynshteyn D, Jeong H. Caffeine induces CYP1A2 expression in rat hepatocytes but not in human hepatocytes. Drug Metab Lett. 2012;6(2):116–9.
Warrington JS, von Moltke LL, Shader RI, Greenblatt DJ. In vitro biotransformation of sildenafil (Viagra) in the male rat: the role of CYP2C11. Drug Metab Dispos. 2002;30(6):655. https://doi.org/10.1124/dmd.30.6.655.
Zhou T, Chen Y, Huang C, Chen G. Caffeine induction of sulfotransferases in rat liver and intestine. J Appl Toxicol. 2012;32(10):804–9. https://doi.org/10.1002/jat.1698.
Abdulla M, Mallah E, Abu Dayyih W, Rayyan W, Elhajji F, Bustami M, et al. Influence of energy drinks on pharmacokinetic parameters of sildenafil in rats. Biomed Pharmacol J. 2018;11:1317–28. https://doi.org/10.13005/bpj/1494.
Mehrotra N, Gupta M, Kovar A, Meibohm B. The role of pharmacokinetics and pharmacodynamics in phosphodiesterase-5 inhibitor therapy. Int J Impot Res. 2007;19(3):253–64. https://doi.org/10.1038/sj.ijir.3901522.
Walker DK. Pharmacokinetics and metabolism of sildenafil in mouse, rat, rabbit, dog and man. Xenobiotica. 1999;29(3):297–310. https://doi.org/10.1080/004982599238687.
Abbott D, Comby P, Charuel C, Graepel P, Hanton G, Leblanc B, et al. Preclinical safety profile of sildenafil. Int J Impot Res. 2004;16(6):498–504. https://doi.org/10.1038/sj.ijir.3901232.
Lin Y-K, Sheu M-T, Tzen T-Z, Ho H-O. Biotransformation of sildenafil in the male rat: evaluation of drug interactions with testosterone and carbamazepine. Drug Dev Ind Pharm. 2008;34(11):1219–26. https://doi.org/10.1080/03639040802005032.
Kot M, Daniel W. Effect of cytochrome P450 (CYP) inducers on caffeine metabolism in the rat. Pharmacol Rep: PR. 2007;59:296–305.
Kot M, Daniel WA. Relative contribution of rat cytochrome P450 isoforms to the metabolism of caffeine: the pathway and concentration dependence. Biochem Pharmacol. 2008;75(7):1538–49. https://doi.org/10.1016/j.bcp.2007.12.017.
Albassam AA, Markowitz JS. An appraisal of drug-drug interactions with green tea (Camellia sinensis). Planta Med. 2017;234(06):496–508.
Knop J, Misaka S, Singer K, Hoier E, Müller F, Glaeser H, et al. Inhibitory effects of green tea and (−)-epigallocatechin Gallate on transport by OATP1B1, OATP1B3, OCT1, OCT2, MATE1, MATE2-K and P-glycoprotein. PLoS One. 2015;10(10):e0139370. https://doi.org/10.1371/journal.pone.0139370.
Oga EF, Sekine S, Shitara Y, Horie T. Pharmacokinetic herb-drug interactions: insight into mechanisms and consequences. Eur J Drug Metab Pharmacokinet. 2016;41(2):93–108. https://doi.org/10.1007/s13318-015-0296-z.
Musther H, Olivares-Morales A, Hatley OJD, Liu B, Rostami HA. Animal versus human oral drug bioavailability: do they correlate? Eur J Pharm Sci. 2014;57:280–91. https://doi.org/10.1016/j.ejps.2013.08.018.
Wang L, Prasad B, Salphati L, Chu X, Gupta A, Hop CECA, et al. Interspecies variability in expression of hepatobiliary transporters across human, dog, monkey, and rat as determined by quantitative proteomics. Drug Metab Dispos. 2015;43(3):367. https://doi.org/10.1124/dmd.114.061580.
Toutain P-L, Ferran A, Bousquet-Mélou A. Species differences in pharmacokinetics and pharmacodynamics. In: Cunningham F, Elliott J, Lees P, editors. Comparative and veterinary pharmacology. Berlin: Springer Berlin Heidelberg; 2010. p. 19–48.
Bae S, Bae SK, Lee B. Effect of hepatic CYP inhibitors on the metabolism of sildenafil and formation of its metabolite, N-desmethylsildenafil, in rats in vitro and in vivo. J Pharm Pharmacol. 2009;61:1637–42. https://doi.org/10.1211/jpp/61.12.0008.
Shin HS, Bae SK, Lee MG. Pharmacokinetics of sildenafil after intravenous and oral administration in rats: hepatic and intestinal first-pass effects. Int J Pharm. 2006;320(1):64–70. https://doi.org/10.1016/j.ijpharm.2006.04.005.
Acknowledgments
The authors would like to thank the entire team of Science and Research Department at Medical University “Prof. Dr. Paraskev Stoyanov” for their support.
Funding
This study was funded by the Science Fund of Medical University “Prof. Dr. Paraskev Stoyanov” (project № 20019).
Author information
Authors and Affiliations
Contributions
MRI performed the literature review and wrote the manuscript. IZ extracted Bancha methylxanthines. MRI carried out the randomization and was aware of the group allocation. MRI, NH, SS and KG conducted the experiments. SS and MRI performed the HPLC analysis. All authors contributed to the design of the study and data analysis. All authors read and approved the final version of the manuscript before submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial and non-financial conflicts of interest to declare.
Ethics approval
The study protocol was approved by the Bulgarian Food Safety Agency and all the requirements for the protection and humane treatment of laboratory animals according to the Directive 2010/63/EU were followed.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Radeva-llieva, M., Stoeva, S., Hvarchanova, N. et al. Influence of methylxanthines isolated from Bancha green tea on the pharmacokinetics of sildenafil in rats. DARU J Pharm Sci 30, 75–84 (2022). https://doi.org/10.1007/s40199-022-00433-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40199-022-00433-z