Abstract
Wastewater contaminated with the antibiotic-resistant bacteria, Pseudomonas aeruginosa, can contribute to human community-acquired infections when released into receiving waters. This study outlines a novel process of phage application that can reduce the reservoir of P. aeruginosa in both primary wastewater (PWW) and secondary wastewater (SWW). The phage PA25 was first successfully isolated from SWW and is a double-stranded DNA phage, classified as a Siphoviridae family as defined by plaque morphology, electron microscopy and host range. Bacteria such as Pseudomonas are the natural host of this virus; the addition of Siphoviridae PA25 has resulted in the greatest reduction of bacteria from unsterilized PWW compared to unsterilized SWW. Experimental results showed a bacterial reduction of 5ULog discharge in PWW compared only 3ULog in SWW. The addition of PA25 to wastewater can also eliminate streptomycin resistance in P. aeruginosa ATCC strain 27853. Infected P. aeruginosa showed decreased resistance to the antibiotics gentamicin and rifampicin.



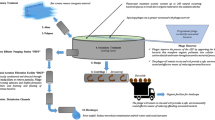
taken from the primary domestic and from the secondary wastewater according to the addition or not of the phage PA25. (Pg): phage PA25, StDW: Sterilized distilled water + P. aeruginosa. StDW + Pg: Sterilized distilled water + P. aeruginosa + Phage PA25 StPWW = Sterilized Primary wastewater + P. aeruginosa StPWW + Pg: Sterilized Primary wastewater + P. aeruginosa + Phage PA25. UstPWW: Unsterilized primary wastewater + P. aeruginosa UstPWW + Pg: Unsterilized primary wastewater + P. aeruginosa + Phage PA25. StSWW = Sterilized Secondary wastewater + P. aeruginosa StSWW + Pg = Sterilized Secondary wastewater + P. aeruginosa + Phage PA25. UstSWW:Unsterilized secondary wastewater + P. aeruginosa UstSWW + Pg Unsterilized secondary wastewater + P. aeruginosa + Phage PA25

Similar content being viewed by others
References
Abedon ST (2020) Phage-phage, phage-bacteria, and phage-environment communication in biocommunication of phages. Springer, pp 23–70
Acar JF (2000) Antibiotic synergy and antagonism. Med Clin North Am 84(6):1391–1406
Ackermann HW (2001) Frequency of morphological phage descriptions in the year 2000. AdvVirol 146(5):843–857
Ackermann HW (2007) 5500 Phages examined in the electron microscope. Arch Virol 152:227–243. https://doi.org/10.1007/s00705-006-0849-1
Ackermann HW (2009) Basic phage electron microscopy. Humana press, pp 113–126
Ackermann HW (2012) Bacteriophage electron microscopy. Adv Virus Res 82:1–32
Ackermann HW, DuBow MS (1987) Viruses of prokaryotes. CRC Press, General properties bacteriophages 1:86
Adams MH (1959) Methods of study of bacterial viruses Bacteriophages. Interscience Publishers
Al-Abri M, Al-Ghafri B, Bora T, Dobretsov S, Dutta J, Castelletto S, Boretti A (2019) Chlorination disadvantages and alternative routes for biofouling control in reverse osmosis desalination. NPJ Clean Water 2(1):1–16
Alisky J, Iczkowski K, Rapoport A, Troitsky N (1998) Bacteriophages show promise as antimicrobial agents. J Infect 36(1):5–15
Aprea G, ARDPrencipe VA, Migliorati G (2015) Bacteriophage morphological characterization by using transmission electron microscopy. J Life Sci 9:214–220
Aprea G, D’Angelantonio D, Boni A, Connerton P, Connerton I, Scattolini S, Marotta F, Pomilio F, Migliorati G, D’Alterio N, Di Giannatale E (2018) Isolation and morphological characterization of new bacteriophages active against campylobacter jejuni. Am J ClinMicrobiolAntimicrob 1(1):1004
Al-Rekabi WS, Qiang H, Qiang WW (2007) Improvements in wastewater treatment technology. Pak J Nutr 6(2):104–110
Armon R, Kott Y (1993) A simple, rapid and sensitive presence/absence detection test for bacteriophage in drinking water. J ApplBacteriol 74(4):490–496
Berglund B (2015) Environmental dissemination of antibiotic resistance genes and correlation to anthropogenic contamination with antibiotics. Infect EcolEpidemiol 5(1):1–28564
Bradbury J (2004) My enemy’s enemy is my friend Using phages to fight bacteria. Lancet 363:624–625
Brizou F (1972) Famille de Pseudomonacae dans « bactériologie médicale ». Editions médicales Flammarison, 469–1523.
Broniewski JM, Meaden S, Paterson S, Buckling A, Westra ER (2020) The effect of phage genetic diversity on bacterial resistance evolution. ISME J 14(3):828–836
Brüssow H, Kutter E (2005) Phage ecology. Bacteriophages BiolAppl 70:129–164
Brussow H, Canchaya C, Hardt WD (2004) Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion. MicrobiolMolBiol 68(3):560–602
Burmeister AR, Fortier A, Roush C, Lessing AJ, Bender RG, Barahman R et al (2020) Pleiotropy complicates a trade-off between phage resistance and antibiotic resistance. Proc Nat Acad Sci 117(21):11207–11216
Cabello FC (2006) Heavy use of prophylactic antibiotics in aquaculture: a growing problem for human and animal health and for the environment. Environ Microbiol 8(7):1137–1144
Cabral JP (2010) Water microbiology bacterial pathogens and water. Int J Environ Res Publ Health 7(10):3657–3703
Campbell EA, Korzheva N, Mustaev A, Murakami K, Nair S, Goldfarb A, Darst SA (2001) Structural mechanism for rifampicin inhibition of bacterial RNA polymerase. Cell 104(6):901–912
Cervantes-Vega C, Chávez J, Rodríguez MG (1986) Antibiotic susceptibility of clinical isolates of Pseudomonas aeruginosa. Antonie Van Leeuwenhoek 52:319–324
Cisek AA, Dąbrowska I, Gregorczyk KP, Wyżewski Z (2017) Phage therapy in bacterial infections treatment: one hundred years after the discovery of bacteriophages. CurrMicrobiol 74(2):277–283
Comeau AM, Tétart F, Trojet SN, Prère MF, Krisch HM (2008) La « synergiephages-antibiotiques » Un enjeu pour la phagothérapie. Médecine/sciences 24(5):449–451
Courvalin P (2001) Resistance aux antibiotiques. SocMicrobiol 45:1007–1031
Danovaro R, Fonda US, Pusceddu A (2009a) Climate change and the potential spreading of marine mucilage and microbial pathogens in the Mediterranean Sea. PLoS ONE 4:e7006. https://doi.org/10.1371/journal.pone.0007006
Danovaro R, Umani SF, Pusceddu A (2009) Climate change and the potential spreading of marine mucilage and microbial pathogens in the Mediterranean Sea. Plos One 4(9):256
Dean CR, Visalli MA, Projan SJ, Sum PE, Bradford PA (2003) Efflux mediated resistance to tigecycline (GAR-936) in Pseudomonas aeruginosa PAO1. Antimicrob Agents Chemother 47(3):972–978
Ebrahimi A, Arvaneh Z, Mahzounieh M, Lotfalian S (2017) Antibiotic resistance induction by benzalkonium chloride exposure in nosocomial pathogens. Int J Infect 4(2):e40296. https://doi.org/10.5812/iji.40296
Gerba CP, Pepper IL (2019) Municipal wastewater treatment in environmental and pollution science. Academic Press
Golkar Z, Bagasra O, Pace DG (2014) Bacteriophage therapy: a potential solution for the antibiotic resistance crisis. J Infect Develop Ctries 8(02):129–136
Kadurugamuwa JL, Clarke AJ, Beveridge TJ (1993) Surface action of gentamicin on Pseudomonas aeruginosa. J Bacteriol 175(18):5798–5805
Kamizoulis G, Saliba L (2004) Development of coastal recreational water quality standards in the Mediterranean. Environ Int 30(6):841–854
Kropinski AM, Mazzocco A, Waddell TE, Lingohr E, Johnson RP (2009) Enumeration of bacteriophages by double agar overlay plaque assay in bacteriophages. Humana Press, pp 69–76
Labrie M, Reguiba N, Tremblay N (2009) ManuelleApprentissage par problèmes à l’Université du Québec à Montréal (UQAM). Point Biolo 3:1–16
León M, Bastías R (2015) Virulence reduction in bacteriophage resistant bacteria. Front Microbiol 6:1–343
Leverentz B, Conway WS, Janisiewicz W, Camp MJ (2004) Optimizing concentration and timing of a phage spray application to reduce Listeria monocytogenes on honeydew melon tissue. J Food Prot 67(8):1682–1686
Levin BR, Bull JJ (1996) Phage therapy revisited: the population biology of a bacterial infection and its treatment with bacteriophage and antibiotics. Am Nat 147(6):881–898
Lister PD, Wolter DJ, Hanson ND (2009) Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. ClinMicrobiol Rev 22(4):582–610
Mansour W (2018) Tunisian antibiotic resistance problems: three contexts but one health. Afr Health Sci 18(4):1202–1203
Merril CR, Scholl D, Adhya SL (2003) The prospect for bacteriophage therapy in Western medicine. Nat Rev Drug Discovery 2(6):489–497
Moghadam MT, Amirmozafari N, Shariati A, Hallajzadeh M, Mirkalantari S, Khoshbayan A, Jazi FM (2020) How phages overcome the challenges of drug resistant bacteria in clinical infections. Infection and Drug Resistance 13:1–45
Nassar MS, Hazzah WA, Bakr WM (2019) Evaluation of antibiotic susceptibility test results: how guilty a laboratory could be? J Egypt Public Health Assoc 94(1):4
Olivares E, Badel-Berchoux S, Provot C, Prévost G, Bernardi T, Jehl F (2020) Clinical impact of antibiotics for the treatment of Pseudomonas aeruginosa biofilm infections. Front Microbiol 10:2894
Petrovski S, Seviour RJ, Tillett D (2011) Genome sequence and characterization of the Tsukamurella bacteriophage TPA2. Appl Environ Microbiol 77(4):1389–1398
Prieto MD, Lopez B, Juanes JA, Revilla JA, Llorca J, Delgado-Rodriguez M (2001) Recreation in coastal waters: health risks associated with bathing in sea water. J Epidemiol Community Health 55(6):442–447
Pukatzki S, Kessin RH, Mekalanos JJ (2002) The human pathogen Pseudomonas aeruginosa utilizes conserved virulence pathways to infect the social amoeba Dictyosteliumdiscoideum. ProcNatlAcadSci 99(5):3159–3164
Rivera-Utrilla J, Sánchez-Polo M, Ferro-García MÁ, Prados-Joya G, Ocampo-Pérez R (2013) Pharmaceuticals as emerging contaminants and their removal from water. Rev Chemos 93(7):1268–1287
Saidi N, Kouki S, Mehri I, Benrajeb A, Bellila A, Abdennaceur H, Ouzari HI (2011) Biofilm and siderophore effects on secondary waste water disinfection. CurrMicrobiol 63:337–340
Schwartz T, Armant O, Bretschneider N, Hahn A, Kirchen S, Seifert M, Dötsch A (2015) Whole genome and transcriptome analyses of environmental antibiotic sensitive and multi-resistant Pseudomonas aeruginosa isolates exposed to waste water and tap water. MicrobBiotechnol 8(1):116–130
Sepúlveda-Robles O, Kameyama L, Guarneros G (2012) High diversity and novel species of Pseudomonas aeruginosa bacteriophages. Appl Environ Microbiol 78(12):4510–4515
Silva YJ, Costa L, Pereira C, Cunha Â, Calado R, Gomes NC, Almeida A (2014) Influence of environmental variables in the efficiency of phage therapy in aquaculture. MicrobBiotechnol 7(5):401–413
Slekovec C, Plantin J, Cholley P, Thouverez M, Talon D, Bertrand X, Hocquet D (2012) Tracking down antibiotic-resistant Pseudomonas aeruginosa isolates in a wastewater network. PloS one 7(12):e49300
Sorum H (2006) Antimicrobial drug resistance in fish pathogens. In: Aarestrup FM (ed) Antimicrobial resistance in bacteria of animal origin. American Society for Microbiology Press, pp 213–238
Sulakvelidze A, Kutter E (2005) Bacteriophage therapy in humans bacteriophages biology and applications. CRC Press
Sulakvelidze A, Morris JG (2001) Bacteriophages as therapeutic agents. Ann Med 33:507–509
Summers WC (2005) Bacteriophage research: early history. Bacteriophages: Biology and applications., pp. 5-27
Tan X, Li H, Li X, Sun W, Jin C, Chen L, Sun C (2020) A novel isophorone wastewater treatment technology-wet electrocatalytic oxidation and its degradation mechanism study. J Hazard Mater 1:122035
Torres-Barceló C, Arias-Sánchez FI, Vasse M, Ramsayer J, Kaltz O, Hochberg ME (2014) A window of opportunity to control the bacterial pathogen Pseudomonas aeruginosa combining antibiotics and phages. PloS one 9(9):1056
Tsamba L, Correc O, Couzinet A (2020) Chlorination by-products in indoor swimming pools: development of a pilot pool unit and impact of operating parameters. Environ Int 137:105566
Van Houte S, Ekroth AK, Broniewski JM, Chabas H, Ashby B, Bondy-Denomy J, Westra ER (2016) The diversity-generating benefits of a prokaryotic adaptive immune system. Nature 532(7599):385–388
Wang Z, Zheng P, Ji W, FuQYan WHY, Sun J (2016) SLPW: A virulent bacteriophage targeting methicillin-resistant Staphylococcus aureus in vitro and in vivo. Front Microbiol 7:1–934
Watts S, Julian TR, Maniura-Weber K, Graule T, Salentinig S (2020) Colloidal transformations in MS2 virus particles: driven by pH, influenced by natural organic matter. ACS Nano 14(2):1879–1887
Withey S, Cartmell E, Avery LM, Stephenson T (2005) Bacteriophages—potential for application in wastewater treatment processes. Sci Total Environ 339(1–3):1–18
Wong CW, Delaquis P, Goodridge L, Lévesque RC, Fong K, Wang S (2020) Inactivation of Salmonellaenterica on post-harvest cantaloupe and lettuce by a lytic bacteriophage cocktail. Curr Res Food Sci 2:25–32
Yee Y, Kisslinger B, Yu V, Jin D (1996) A mechanism of rifamycin inhibition and resistance in Pseudomonas aeruginosa. Antimicrob Chemoth 38:133–137
Yuan Y, Qu K, Tan D, Li X, Wang L, Cong C, Xu Y (2019) Isolation and characterization of a bacteriophage and its potential to disrupt multi-drug resistant Pseudomonas aeruginosa biofilms. MicrobPathog 128:329–336
Zhang L, Mah TF (2008) Involvement of a novel efflux system in biofilm-specific resistance to antibiotics. J Bacteriol 190(13):4447–4452
Acknowledgments
This study was supported by a grant from Tunisian Ministry of Higher Education and Scientific Research in the ambit of LR19CERTE04 (2o19_2022 Programs). Authors are Grateful for technical team of CERTE, particularly to Miss Nessrine Chourabi. We thank Pr. Sylvain Moineau and Pr Josée Harel and members of their teams for discussion at the beginning of the project and for phage identification. Also, authors are grateful for Pr Steven Aust for revision and English improvement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Editorial Responsiblity: Parveen Fatemeh Rupani.
Rights and permissions
About this article
Cite this article
Grami, E., Salhi, N., Sealey, K.S. et al. Siphoviridae bacteriophage treatment to reduce abundance and antibiotic resistance of Pseudomonas aeruginosa in wastewater. Int. J. Environ. Sci. Technol. 19, 3145–3154 (2022). https://doi.org/10.1007/s13762-021-03366-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-021-03366-3