Abstract
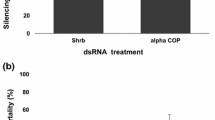
The “cotton boll weevil” (Anthonomus grandis Boheman) is a key pest in America whose larval stage develops within the cotton flower bud. During its development, the larva uses the flower bud as food and as a shelter from predators. This behavior limits the effective control through conventional insecticide applications and biocontrol techniques. Increasing genetic information from insects has allowed the development of new control technologies based on the use of RNA interference (RNAi) to design orally delivered double-stranded RNA (dsRNA) strategies. In this study, we evaluated the effect of continuous oral administration of six specific dsRNA in order to identify an effective target gene for RNAi-mediated control of cotton boll weevil. First, six selected A. grandis gene fragments were amplified and cloned to perform in vivo synthesis of the specific dsRNA, and subsequently, larvae and adults were fed with this dsRNA for 2 weeks. Larvae mortality ranged from 40 to 60% depending on the targeted gene sequence. Indeed, α-amylase and cytochrome p450 dsRNAs were the most effective. Oral administration in adults caused smaller but still significant death rates (15–30%). Thus, the results demonstrated RNAi responses depend on life stages and target genes. The dsRNA ingestion was capable of providing knockdown mRNA levels in cotton boll weevil midgut and this effect was significantly higher in the larval stage. In this study, we present a new report of silencing of midgut genes in A. grandis larva induced by continuously feeding with dsRNA. This potential new tool should be further evaluated in cotton boll weevil control strategies.




Similar content being viewed by others
References
Almeida Garcia R, Lima Pepino Macedo L, Cabral Do Nascimiento D, Gillet F, Moreira-Pepino C, Faheem M, Moreschi Basso A, Mattar Silva M, Grossi De Sa M (2007) Nucleases as a barrier to gene silencing in the cotton boll weevil, Anthonomus grandis. PLoS One 12:e0189600
Araujo R, Santos A, Pinto F, Gontijo N, Lehane M, Pereira M (2006) RNA interference of the salivary gland nitrophorin 2 in the triatomine bug Rhodnius prolixus (Hemiptera: Reduviidae) by dsRNA ingestion or injection. Insect Biochem Mol Biol 36:683–693
Baum J, Bogaert T, Clinton W, Heck G, Feldmann P, Ilagan O, Johnson S, Plaetinck G, Munyikwa T, Pleau M, Vaughn T, Roberts J (2007) Control of coleopteran insect pests through RNA interference. Nat Biotechnol 25:1322–1326
Chen W, Zhang Q, Kong J, Hu F, Li B, Wu C, Qin C, Zhang P, Shi N, Hong Y (2015) MR VIGS: microRNA-based virus-induced gene silencing in plants. Methods Mol Biol 1287:147–157
Cross W, Lukefahr M, Fryxell P, Burke H (1975) Host plants of the boll weevil. Environ. Entomol. 4: 19-26. Environ Entomol 4:19–26
Cuadrado GA (2002) Anthonomus grandis Boheman (Coleoptera: Curculionidae) en la Zona Central y Sur Oeste de Misiones, Argentina: Polen Como Fuente Alimenticia y su Relación con el Estado Fisiológico en Insectos Adultos. Neotrop Entomol. 31:121–132
Dias S, Franco O, Magallanes CP, De Oliveira-Neto O, Laumann R, Figueira E, Melo F, Grossi De Sa MF (2005) Molecular cloning and expression of an alpha-amylase inhibitor from rye with potential for controlling insect pests. Protein J 24:113–123
Fire AX, Montgomery MK, Kostas SA, Driver SE, Mello CC (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391:806–811
Firmino AA, Fonseca FC, De Macedo LL, Coelho RR, Antonio De Souza J, Togawa RC, Silva-Junior OB, Pappas J, Da Silva MC, Engler G, Grossi De Sa MF (2013) Transcriptome analysis in cotton boll weevil (Anthonomus grandis) and RNA interference in insect pests. PLoS One 8:e85079
Fye RE, Cole CL, Bull DL (1970) Populations of boll weevil in selected felds in Presidio County, Texas, and Ojinaga, chiauahua, Mexico, in late 1968 subsequent to reproductive-diapause control program in 1965-1967. J Econ Entomol 63:1084–1086
Gamboa Cedeño A, Niz J, Sciocco de Cap A, Salvador R (2015) Double-stranded RNA synthesized in bacteria can be transferred to bee and varroa tissues. J Apic Res 54:2:99-100
Garralla GA (2000) Plantas Alimenticias Alternativas del Picudo del Algodonero (Anthonomus grandis Boh.) (Coleoptera: Curculionidae) en la Provincia de Formosa, Argentina. Análisis Palinológico del Tracto Digestivo. An Soc Entomol Brasil 29:245–255
Gillet FX, Garcia RA, Macedo LL, Albuquerque EV, Silva MC, Grossi Da Silva MF (2017) Investigating engineered ribonucleoprotein particles to improve oral RNAi delivery in crop insect pests. Front Physiol 8:256
Hopkins AR, Taft HM, James W (1975) Reference LC50 values for some insecticides against the boll weevil. J Econ Entomol 68:189–192
Huvenne H, Smagghe G (2010) Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: a review. J Insect Physiol 56:227–235
Joga MR, Zotti M, Smaghee G, Christiaens O (2016) RNAi efficiency, systemic properties, and novel delivery methods for pest insect control: what we know so far. Front Physiol 7:553
Kanga LH, Plapp FW, Wall ML, Karner MA, Huffman RL, Fuchs TW, Elzen GW, Martinez-Carillo JL (1995) Monitoring tolerance to insecticides in boll weevil populations (Coleoptera: Curculionidae) from Texas, Arkansas, Oklahoma, Mississippi, and Mexico. J Econ Entomol 88:198–204
Lecuona R (2009) Cría masiva en laboratorio del picudo del algodonero Anthonomus grandis Boheman (Coleoptera:Curculionidae),. En: Actas de XIII Jornadas Fitosanitarias Argentinas. Termas de Rio Hondo, S. del Estero,Argentina., pp. Z-45
Li J, Chen Q, Lin Y, Jiang T, Wu G, Hua H (2011a) RNA interference in Nilaparvata lugens (Homoptera: Delphacidae) based on dsRNA ingestion. Pest Manag Sci 67:852–859
Li X, Zhang M, Zhang H (2011b) RNA interference of four genes in adult Bactrocera dorsalis by feeding their dsRNAs. PLoS One 6:e17788
Livak K, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2 (−ΔΔ C(T)) method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Macedo L, Souza A, Coelho R, Fonseca F, Firmino A, Silva M, Fragoso R, Albuquerque E, Silva M, Almeida Engle J, Grossi Da Silva FM (2017) Knocking down chitin synthase 2 by RNAi is lethal to the cotton boll weevil. Biotech Res and Innov 1(1, January–December):72–86
Mao YB, Wnah J, Hong GJ, Tao X, Wang L, Huang LJ, Chen XY (2007) Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat Biotechnol 25:1307–1313
Morales LP, Mendes Neto SF, Costa F, Oliveira T (1997) Resistência de genótipos de algodoeiro a Anthonomus grandis Boh Frankliniella sp e Aphis gossypii Glover. An Soc Entomol Brasil 26:93–98
Nunes FM, Simoes ZL (2009) A non-invasive method for silencing gene transcription in honeybees maintained under natural conditions. Insect Biochem Mol Biol 39:157–160
Oliveira-Neto OB, Batista JA, Rigden DJ, Franco O, Falcao R, Fragoso RR, Mello LV, Dos Santos R, Da Grossi SAMF (2003) Molecular cloning of alpha-amylases from cotton boll weevil, Anthonomus grandis and structural relations to plant inhibitors: an approach to insect resistance. J Protein Chem 22:77–87
Philip BN, Tomoyasu Y (2011) Gene knockdown analysis by double-stranded RNA injection. Methods Mol Biol 772:471–497
Rangasamy M, Siegfried BD (2012) Validation of RNA interference in western corn rootworm Diabrotica virgifera virgifera LeConte (Coleoptera: Chrysomelidae) adults. Pest Manag Sci 68:587–591
Salvador R, Principi D, Berretta M, Fernandez P, Paniego N, Sciocco de Cap A, Hopp E (2014) Transcriptomic survey of the midgut of Anthonomus grandis (Coleoptera: Curculionidae). J Insect Sci 14:219
Scharf ME, Schwinghammer MA (2008) Application of RNA interference in functional genomics studies of a social insect. Methods Mol Biol 442:205–229
Showler AT (2007) Subtropical boll weevil ecology. Am Entomol 53:240–249
Terenius O, Papanicolaou A, Garbutt JS, Eleftherianos I, Huvenne H, Kanginakudru S, Albrechtsen M, An C, Aymeric JL, Barthel A, Bebas P, Bitra K, Bravo A, Chevalier F, Collinge DP, Crava CM, de Maagd RA, Duvic B, Erlandson M, Faye I, Felföldi G, Fujiwara H, Futahashi R, Gandhe AS, Gatehouse HS, Gatehouse LN, Giebultowicz JM, Gómez I, Grimmelikhuijzen CJP, Groot AT, Hauser F, Heckel DG, Hegedus DD, Hrycaj S, Huang L, Hull JJ, Iatrou K, Iga M, Kanost MR, Kotwica J, Li C, Li J, Liu J, Lundmark M, Matsumoto S, Meyering-Vos M, Millichap PJ, Monteiro A, Mrinal N, Niimi T, Nowara D, Ohnishi A, Oostra V, Ozaki K, Papakonstantinou M, Popadic A, Rajam MV, Saenko S, Simpson RM, Soberón M, Strand MR, Tomita S, Toprak U, Wang P, Wee CW, Whyard S, Zhang W, Nagaraju J, ffrench-Constant RH, Herrero S, Gordon K, Swevers L, Smagghe G (2011) RNA interference in Lepidoptera: an overview of successful and unsuccessful studies and implications for experimental design. J Insect Physiol 57:231–245
Teague T, Cate J R, Plapp F W (1983) Toxicity of azinphosmethyl and methyl parathion to three populations of boll weevil. Southwest Entomol 8:107–112
Tian H, Peng H, Yao Q, Chen H, Xie Q, Tang B, Zhang W (2009) Developmental control of a lepidopteran pest Spodoptera exigua by ingestion of bacteria expressing dsRNA of a non-midgut gene. PLoS One 13;4(7)
Timmons L, Court DL, Fire A (2001) Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263:103–112
Upadhyay SK, Chandrashekar K, Thakur N, Verma PC, Borgio JF, Singh PK, Tuli R (2011) RNA interference for the control of whiteflies (Bemisia tabaci) by oral route. J Biosci 36:153–161
Vatanparast M, Kazzazi M, Mirzaie-Asl A, Hosseininaveh V (2017) RNA interference-mediated knockdown of some genes involved in digestion and development of Helicoverpa armigera. Bull Entomol Res 9:1–14
Walshe DP, Lehane SM, Lehane MJ, Haines LR (2009) Prolonged gene knockdown in the tsetse fly Glossina by feeding double stranded RNA. Insect Mol Biol 18:11–19
Whyard S, Singh AD, Wong S (2009) Ingested double-stranded RNAs can act as species-specific insecticides. Insect Biochem Mol Biol 39:824–832
Zhang X, Liu X, Ma J, Zhai J (2013) Silencing of cytochrome P450 CYP6B6 gene of cotton bollworm (Helicoverpa armigera) by RNAi. Bull Entomol Res 103:584–591
Zhang J, Khan SA, Hasse C, Ruf S, Heckel DG, Bock R (2015) Pest control. Full crop protection from an insect pest by expression of long double-stranded RNAs in plastids. Science 347:991–994
Zhao YY, Yang G, Wang-Pruski G, You MS (2008) Phyllotreta striolata (Coleoptera: Chrysomelidae): arginine kinase cloning and RNAi-based pest control. Eur J Entomol 105:815–822
Zhou X, Scharf ME (2008) RNA interference in the termite Reticulitermes flavipes through ingestion of double-stranded RNA. Insect Biochem Mol Biol 38:805–815
Acknowledgments
We thank Julia Sabio y García for assistance with the English edition. Addgene Inc. (Cambridge, MA, USA) provided the plasmid L4440, whereas Caenorhabditis Genetics Center (CGC, Minneapolis, MN, USA), which is funded by the NIH National Center for Research Resources (NCCR), kindly provided the bacterial strain HT115(DE3). Most of the work was supported by a joint venture project entitled “Knowledge generation and development of non-pollutant biotechnologies for the control of cotton weevil” signed between INTA and the governments of Chaco, Formosa, Santa Fe, and Santiago del Estero provinces (Argentina).
Funding
Most of the work was supported by a joint venture project entitled “Knowledge generation and development of non-pollutant biotechnologies for the control of cotton weevil” signed between the governments of Chaco, Formosa, Santa Fe, and Santiago del Estero provinces (Argentina) and the National Institute of Agricultural Technology (INTA).
Author information
Authors and Affiliations
Contributions
Conceptualization: Salvador, Ricardo; Niz, José; Pedarros, Analia; Nakaya, Pablo; and Hopp, Esteban. Investigation: Salvador, Ricardo; Niz, José; Pedarros, Analía; and Nakaya, Pablo. Supervision: Salvador, Ricardo and Hopp, Esteban. Writing original draft: Salvador, Ricardo. Writing review and editing: Salvador, Ricardo and Hopp, Esteban. Funding acquisition: Hopp, Esteban.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Edited by Herbert AA Siqueira – UFRPE
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Salvador, R., Niz, J.M., Nakaya, P.A. et al. Midgut Genes Knockdown by Oral dsRNA Administration Produces a Lethal Effect on Cotton Boll Weevil. Neotrop Entomol 50, 121–128 (2021). https://doi.org/10.1007/s13744-020-00819-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-020-00819-1