Abstract
A convenient one-pot two-step strategy for synthesis of the novel tricyclic system 9-chloro-4-methyl-6,11-dihydro-5H-benzo[b]pyrimido[4,5-e][1,4]diazepine have been synthesized through the heterocyclization reaction of 2,4-dichloro-5-(chloromethyl)-6-methylpyrimidine with 4-chloro-o-phenylenediamine under basic conditions. Various derivatives were obtained via treatment with secondary amines. Also, the inhibitory effects of the synthesized compound against bromodomain-containing protein 4 (BRD4) have been evaluated by molecular docking. The results reveal that among the studied compounds (5f) is a more potent inhibitor against this enzyme.
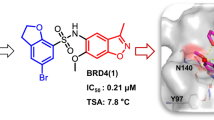
Graphical abstract









Similar content being viewed by others
References
G. Dou, D. Shi, One pot synthesis of cis-bispyrimidodiazepinone derivatives via low-valent titanium reagent (TiCl 4/Sm). Org. Biomol. Chem. 9(20), 7065–7070 (2011). https://doi.org/10.1039/C1OB05791J
Y. Malki, J. Martinez, N. Masurier, 1, 3-Diazepine: a privileged scaffold in medicinal chemistry. Med. Res. Rev. (2021). https://doi.org/10.1002/med.21795
M.A. Rashid et al., 1, 4-diazepines: a review on synthesis, reactions and biological significance. Curr. Org. Synth. 16(5), 709–729 (2019). https://doi.org/10.2174/1570179416666190703113807
E.Y. Zelina et al., A Route to (Het) arene-annulated pyrrolo [1, 2-d][1, 4] diazepines via the expanded intramolecular paal-knorr reaction: nitro group and furan ring as equivalents of amino group and 1, 4-diketone. J. Org. Chem. 84(21), 13707–13720 (2019). https://doi.org/10.1021/acs.joc.9b01925
H. Farhid et al., Multicomponent reactions as a potent tool for the synthesis of benzodiazepines. Org. Biomol. Chem. (2021). https://doi.org/10.1039/D0OB02600J
J. Li et al., Design, synthesis, and biological evaluation of novel 4, 4-difluoro-1-methyl-N, 6-diphenyl-5, 6-dihydro-4 H-pyrimido [4, 5-b][1, 2, 4] triazolo [4, 3-d][1, 4] diazepin-8-amine derivatives as potential BRD4 inhibitors. Chem. Biol. Drug Des. 97(5), 1117–1128 (2021). https://doi.org/10.1111/cbdd.13833
M. Pozarentzi, J. Stephanidou-Stephanatou, C.A. Tsoleridis, An efficient method for the synthesis of 1, 5-benzodiazepine derivatives under microwave irradiation without solvent. Tetrahedron Lett. 43(9), 1755–1758 (2002). https://doi.org/10.1016/S0040-4039(02)00115-6
J.H. Song et al., Formation of benzodiazepines and pyrazinylquinoxalines from aromatic and heteroaromatic ketones via deoximation. Asian J Chem 32, 1676–1680 (2020)
A. Di Capua, A. Reale, M. Paolino, G. Chemi, S. Brogi, A. Cappelli, M. Anzini, Design, synthesis and biological evaluation of 7-substituted 4-phenyl-6H-imidazo [1, 5-a] thieno [3, 2-f][1, 4] diazepines as safe anxiolytic agents. Eur J Med Chem 200, 112405 (2020). https://doi.org/10.1016/j.ejmech.2020.112405
K. Basavaraja, V. Vaidya, C. Chandrashekhar, Synthesis of Benzofuro [3, 2-e]-1, 4-diazepines of Pharmacological Interest. E-J Chem 5(3), 567–571 (2008)
J. Yang et al., Synthesis of tricyclic 4-chloro-pyrimido [4, 5-b][1, 4] benzodiazepines. Org. Lett. 7(8), 1541–1543 (2005). https://doi.org/10.1021/ol050181f
B. Insuasty et al., Synthesis of 1-Benzyl-6-(4-chlorophenyl)-2-(4-R-phenyl)-4-(4-Rstyryl)-2, 3-dihydropyrazolo [3, 4-b][1, 4] diazepines. Molecules 6(8), 710–715 (2001). https://doi.org/10.3390/60800710
D.A. Horton, G.T. Bourne, M.L. Smythe, The combinatorial synthesis of bicyclic privileged structures or privileged substructures. Chem. Rev. 103(3), 893–930 (2003). https://doi.org/10.1021/cr020033s
H.R. Qomi, A. Habibi, Synthesis of a novel functionalized tricyclic pyrimidine-fused 1, 5-benzodiazepine library. Tetrahedron 73(21), 2991–3001 (2017). https://doi.org/10.1016/j.tet.2017.03.079
E. Batlle, E. Lizano, M. Viñas, M. D. Pujol 1, 4-Benzodiazepines and new derivatives: description, analysis, and organic synthesis. Med Chem pp. 63–90 (2019)
G.C. Brahmbhatt et al., New pyrazolyl-dibenzo [b, e][1, 4] diazepinones: room temperature one-pot synthesis and biological evaluation. Mol. Diversity 24(2), 355–377 (2020). https://doi.org/10.1007/s11030-019-09958-z
M. Jose, M. Nogueras, J. Cobo, Straightforward synthesis of pyrimido [4, 5-e][1, 4] diazepines via 6-aminopyrimidin-5-carbaldehydes. Arab. J. Chem. 12(8), 4579–4595 (2019). https://doi.org/10.1016/j.arabjc.2016.07.012
Y. Hu et al., BRD4 inhibitor inhibits colorectal cancer growth and metastasis. Int. J. Mol. Sci. 16(1), 1928–1948 (2015). https://doi.org/10.3390/ijms16011928
T.A. Stroganova et al., A new strategy for pyrrolo [1, 2-a][1, 4] diazepine structure formation. Synlett 2007(07), 1106–1108 (2007). https://doi.org/10.1055/s-2007-977430
F. Heaney, C. Burke, D. Cunningham, P. McArdle, Pyrimidine annelated heterocycles—synthesis and cycloaddition of the first pyrimido [1, 4] diazepine N-oxides. J Chem Soc Perkin Trans 1(6), 622–632 (2001). https://doi.org/10.1039/B007163N
H.B. Tan, Y.F. Wang, J. Xu, C.S. Hu, One-pot, multi-component synthesis of ethyl (E)-1-(3-ethoxy-3-oxoprop-1-en-1-yl)-2-aryl-2, 5-dihydro-1H-benzo [b][1, 4] diazepine-3-carboxylate derivatives. Tetrahedron Lett 61(11), 151604 (2020). https://doi.org/10.1016/j.tetlet.2020.151604
M. Zia, M. Khalid, S. Hameed, E. Irran, M.M. Naseer, Synthesis and solid state self-assembly of a 1, 4-diazepine derivative: Water cluster as molecular glue and conformational isomerism. J Mol Struct 1207, 127811 (2020). https://doi.org/10.1016/j.molstruc.2020.127811
S. Sheikhi-Mohammareh, M. Mashreghi, A. Shiri, Robust approach leading to novel densely functionalized four-cyclic benzo [e] pyrazolo [5′, 1′: 2, 3] pyrimido [4, 5-b][1, 4] diazepines with antibacterial activity toward resistant strains. J. Iran. Chem. Soc. 17(7), 1555–1566 (2020). https://doi.org/10.1007/s13738-020-01875-5
R. Nongrum, G.K. Kharmawlong, J.W.S. Rani, N. Rahman, A. Dutta, R. Nongkhlaw, Organocatalytic green approach towards the fabrication of fused benzo N, N-containing heterocycles facilitated by ultrasonic irradiation. J Heterocycl Chem 56(10), 2873–2883 (2019). https://doi.org/10.1002/jhet.3680
S.K. Maury et al., A facile and efficient multicomponent ultrasound-assisted “on water” synthesis of benzodiazepine ring. Mol. Diversity 25(1), 131–142 (2021). https://doi.org/10.1007/s11030-019-10031-y
J. Gour, S. Gatadi, V. Pooladanda, S.M. Ghouse, S. Malasala, Y.V. Madhavi, S. Nanduri, Facile synthesis of 1, 2, 3-triazole-fused indolo-and pyrrolo [1, 4] diazepines, DNA-binding and evaluation of their anticancer activity. Bioorganic Chem 93, 103306 (2019). https://doi.org/10.1016/j.bioorg.2019.103306
S. Batinac et al., Synthesis of the novel bicyclic oxepinopyrimidine and fluorinated pyrrolidinopyrimidines. Heterocycl-Sendai Inst Heterocycl Chem 63(11), 2523–2536 (2004)
M. Akbarzadeh et al., Synthesis of dihydrobenzo [b] pyrimido [4, 5-e][1, 4] thiazepines; derivatives of a novel ring system. J. Chem. Res. 39(9), 531–534 (2015). https://doi.org/10.3184/174751915X14401726334645
S. Maiti et al., Synthesis of 1, 4-diazepanes and benzo [b][1, 4] diazepines by a domino process involving the in situ generation of an aza-Nazarov reagent. J. Org. Chem. 85(18), 11924–11933 (2020). https://doi.org/10.1021/acs.joc.0c01774
P.D. Landor, H.N. Rydon, Polyazanaphthalenes Part II. Attempted synthesis of some analogues of pteroic acid. J Chem Soc (Resumed) (1955). https://doi.org/10.1039/JR9550001113
Acknowledgements
We gratefully appreciate Ferdowsi university of the Mashhad Research council for their financial support of this work (grant: 3/53924).
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Assadzadeh, F., Afrough, T., Eshghi, H. et al. A convenient one-pot synthesis of novel heterocyclic systems of 9-chloro-4-methyl-6,11-dihydro-5H-benzo[b]pyrimido[4,5-e][1,4]diazepine and inhibitory evaluation against bromodomain-containing protein 4 (BRD4) enzyme by molecular docking. J IRAN CHEM SOC 20, 2841–2848 (2023). https://doi.org/10.1007/s13738-023-02880-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-023-02880-0