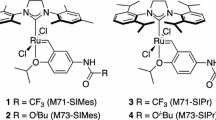
Abstract
A number of natural and biologically active compounds can be synthesized via ruthenium catalysis. As ruthenium is the cheapest noble metal and can be extensively used to synthesize a variety of catalysts, particular attention has been made in the synthesis of chemotherapeutic agents, polymers, biopolymers and agrochemicals through this catalysis. Ruthenium-based catalysts are well famous because of their broad range of functional group, air and moisture tolerance. Different organic reactions such as alkylation, arylation, isomerization and olefin metathesis are efficiently carried out in the presence of these catalysts within short reaction time. In 1992, Robert H. Grubbs first time introduced ruthenium-based catalyst, known as Grubbs first-generation catalyst (G-1) to carry out olefin metathesis effectively. Later on, Grubbs second-generation G-2, third-generation catalyst G-3 and Hoveyda–Grubbs catalysts such as HG-1 and HG-2 were also obtained by changing different ligands with ruthenium. In this review, recent advances in the synthesis of ruthenium-based Grubbs catalysts and their usage in natural product synthesis via olefin metathesis are discussed briefly.
















































































Similar content being viewed by others
References
Q. Yao, A soluble polymer-bound ruthenium carbene complex: A robust and reusable catalyst for ring-closing olefin metathesis. Angew. Chem. Ed. 39, 3896–3898 (2000). https://doi.org/10.1002/1521-3773(20001103)39:21
R.H. Grubb, Olefin metathesis. Tetrahedron 60, 7117–7140 (2004). https://doi.org/10.1016/j.tet.2004.05.124
A.K. Chatterjee, T.L. Choi, D.P. Sanders, R.H. Grubbs, A general model for selectivity in olefin cross metathesis. J. Am. Chem. Soc. 125, 11360–11370 (2003). https://doi.org/10.1021/ja0214882
J.G. Boiteau, P. Van de Weghe, J. Eustache, A new, ring closing metathesis-based synthesis of (−)-Fumagillol. Org Lett 3, 2737–2740 (2001). https://doi.org/10.1021/ol016343
Y. Chen, M.M. Abdellatif, K. Nomura, Olefin metathesis polymerization: Some recent developments in the precise polymerizations for synthesis of advanced materials (by ROMP, ADMET). Tetrahedron 74, 619–643 (2018). https://doi.org/10.1016/j.tet.2017.12.041
R. Akhtar, A.F. Zahoor, B. Parveen, M. Suleman, Development of environmental friendly synthetic strategies for Sonogashira cross coupling reaction: an update. Synth. Commun. 49, 167–192 (2019). https://doi.org/10.1080/00397911.2018.1514636
R. Akhtar, A.F. Zahoor, Transition metal catalyzed Glaser and Glaser-Hay coupling reactions: scope, classical/green methodologies and synthetic applications. Synth. Commun. 50, 3337–3368 (2020). https://doi.org/10.1080/00397911.2020.1802757
M. Yousaf, A.F. Zahoor, R. Akhtar, M. Ahmad, S. Naheed, Development of green methodologies for Heck, Chan-Lam, Stille and Suzuki cross-coupling reactions. Mol. Divers 24, 821–839 (2020). https://doi.org/10.1007/s11030-019-09988-7
I. Munir, A.F. Zahoor, N. Rasool, S.A.R. Naqvi, K.M. Zia, R. Ahmad, Synthetic applications and methodology development of Chan-Lam coupling: a review. Mol. Divers 23, 215–259 (2019). https://doi.org/10.1007/s11030-018-9870-z
M. Westhus, E. Gonthier, D. Brohm, R. Breinbauer, An efficient and inexpensive scavenger resin for Grubbs’ catalyst. Tetrahedron Lett. 45, 3141–3142 (2004). https://doi.org/10.1016/j.tet.2004.02.083
M. Arisawa, A. Nishida, M. Nakagawa, Preparation of nitrogen-containing heterocycles using ring-closing metathesis (RCM) and its application to natural product synthesis. J. Org. Chem. 691, 5109–5121 (2006). https://doi.org/10.1016/j.jorganchem.2006.08.009
P. Schwab, R.H. Grubbs, J.W. Ziller, Synthesis and applications of RuCl2 (CHR’)(PR3)2: the influence of the alkylidene moiety on metathesis activity. J. Am. Chem. Soc. 118(1), 100–110 (1996). https://doi.org/10.1021/ja952676d
C.W. Lee, R.H. Grubbs, Stereoselectivity of macrocyclic ring-closing olefin metathesis. Org. Lett. 2, 2145–2147 (2000). https://doi.org/10.1021/ol006059s
H. Park, H.K. Lee, E.H. Kang, T.L. Choi, Controlled cyclopolymerization of 4,5-disubstituted 1,7-octadiynes and its application to the synthesis of a dendronized polymer using Grubbs catalyst. J. Polym. Sci. A Polym. Chem. 53, 274–279 (2015). https://doi.org/10.1002/pola.27317
R. Bujok, M. Bieniek, M. Masnyk, A. Michrowska, A. Sarosiek, H. Stepowska, K. Grela, Ortho- and para- substituted Hoveyda-Grubbs carbenes. An improved synthesis of highly efficient metathesis initiators. J. Org. Chem. 69, 6894–6896 (2004). https://doi.org/10.1021/jo049222w
J. Yu, Q. He, G. Yang, W. Zhou, Z. Shao, M. Ni, Recent advances and prospective in ruthenium-based materials for electrochemical water splitting. ACS Catal. 9, 9973–10011 (2019). https://doi.org/10.1021/acscatal.9b02457
O.M. Ogba, N.C. Warner, D.J. O’Leary, R.H. Grubbs, Recent advances in ruthenium-based olefin metathesis. Chem. Soc. Rev. 47, 4510–4544 (2018). https://doi.org/10.1039/C8CS00027A
G.C. Vougioukalakis, R.H. Grubbs, Ruthenium-based heterocyclic carbene-coordinated olefin metathesis catalysts. Chem. Rev. 110, 1746–1787 (2010). https://doi.org/10.1021/cr9002424
E. Sundby, L. Perk, T. Anthonsen, A.J. Aasen, T.V. Hansen, Synthesis of (+)-goniothalamin and its enantiomer by combination of lipase catalyzed resolution and alkene metathesis. Tetrahedron 60, 521–524 (2004). https://doi.org/10.1016/j.tet.2003.10.102
S.T. Nguyen, R.H. Grubbs, Synthesis and activities of new single-component, ruthenium-based olefin metathesis catalysts. J. Am. Chem. Soc. 11, 9858–9859 (1993). https://doi.org/10.1021/ja00074a086
R. Dorta, R.A. Kelly, S.T. Nolan, Cross metathesis allowing the conversion of a ruthenium idenylidene complex into Grubbs’ catalyst. Adv. Synth. Catal. 346, 917–920 (2004). https://doi.org/10.1002/adsc.200404047
P. Schwab, M.B. France, J.W. Ziller, R.H. Grubbs, A series of well-defined metathesis catalysts synthesis of [RuCl2(=CHRʹ)(PR3)2] and its reactions. Angew. Chem. Int. Ed. Engl. 34, 2039–2041 (1995). https://doi.org/10.1002/anie.199520391
M.A.O. Volland, B.F. Straub, I. Gruber, F. Rominger, P. Hofmann, Facile synthesis of a ruthenium carbene complex with a cis-chelating diphosphinoethane ligand. J. Org. Chem. 617–618, 288–291 (2001)
R.H. Grubbs, S. Chang, Recent advances in olefin metathesis and its application in organic synthesis. Tetrahedron 54, 4413–4450 (1998). https://doi.org/10.1016/s0040-4020(97)10427-6
M. Scholl, S. Ding, C.W. Lee, R.H. Grubbs, Synthesis and activity of a new generation of ruthenium-based olefin metathesis catalysts coordinated with 1,3-dimesityl-4,5-dihydroimidazol-2-ylidene ligands. Org. Lett. 1, 953–956 (1999). https://doi.org/10.1021/ol990909q
A.M. McKinty, C. Laund, D.W. Stephan, A tridentate-dithiolate ruthenium alkylidene complex: an olefin metathesis catalyst activated by BCl3. Organometallics (2013). https://doi.org/10.1021/om400794u
G.O. Wilson, K.A. Porter, H. Weissman, S.R. White, N.R. Sottos, J.S. Moore, Stability of second generation Grubbs’ alkylidenes to primary amines: formation of novel ruthenium-amine complex. Adv. Synth. Catal. 351, 1817–1825 (2009). https://doi.org/10.1002/adsc.200900134
T. Wang, Q. Xie, W. Guo, S. Wu, H. Zhang, Synthesis and evaluation of naphthalene-1,8-dithiolate chelating ruthenium carbene catalyst for Z-stereoretentive olefin metathesis. J. Org. Chem. 880, 62–67 (2019). https://doi.org/10.1016/j.jorganchem.2018.10.035
A. Hryniewicka, A. Kozlowska, S. Witkowski, New nitrochromenylmethylidene-containing ruthenium metathesis catalyst. J. Org. Chem. 701, 87–92 (2012). https://doi.org/10.1016/j.jorganchem.2011.12.024
H. Hennig, Aqueous-phase organometallic catalysis-concepts and applications. Z. Phys. Chem. 213, 114–115 (1999). https://doi.org/10.1524/zpch.1999.213
S.H. Hong, R.H. Grubbs, Highly active water-soluble olefin metathesis catalyst. J. Am. Chem. Soc. 128, 3508–3509 (2006)
J.O. Krause, O. Nuyken, K. Wurst, Buchmeiser, Synthesis and activity of homogeneous and heterogeneous ruthenium-based metathesis catalysts containing electron-withdrawing ligands. Chem. Eur. J. 10, 777–784 (2004). https://doi.org/10.1002/chem.200305031
A.R. Hlil, S. Moncho, R. Tuba, K. Elsaid, G. Szarka, E.N. Brothers, R.H. Grubbs, M. Al-Hashimi, H.S. Bazzi, Synthesis and catalytic activity of supported acenaphthoimidazolylidene N-heterocyclic carbene ruthenium complex for ring closing metathesis (RCM) and ring opening metathesis polymerization (ROMP). J. Catal. 344, 100–107 (2016). https://doi.org/10.1016/j.jcat.2016.08.019
E. Borré, F. Caijo, C. Crévisy, M. Mauduit, New library of aminosulfonyl-tagged Hoveyda-Grubbs type complexes: synthesis, kinetic studies and activity in olefin metathesis transformations. Beilstein J. Org. Chem. 6, 1159–1166 (2010). https://doi.org/10.3762/bjoc.6.132
J.A. Love, J.P. Morgan, T.M. Trnka, R.H. Grubbs, A practical and highly active ruthenium-based catalyst that effects the cross metathesis of acrylonitrile. Angew. Chem. Int. Ed. 41, 4207–4209 (2002). https://doi.org/10.1002/1521-3757(20021104)114:21%3c4207::AID-ANGE4207%3e3.0.CO;2-G
H. Zhang, Y. Yao, R. Sun, C. Sun, F. Liu, Y. Liu, M. Guo, S. Wang, K. You, Thermally stable pseudo-third-generation Grubbs ruthenium catalysts with pyridine-phosphinimine ligand. Catal. Commun. 49, 43–46 (2014). https://doi.org/10.1016/j.catcom.2014.01.033
J. Balogh, A.R. Hlil, H.L. Su, Z. Xi, H.S. Bazzi, J.A. Gladysz, An analogue of Grubbs third-generation catalyst with fluorophilic pyridine ligands: fluorous/organic phase-transfer activation. ChemCatChem 8, 125–128 (2016). https://doi.org/10.1002/cctc.201500913
D. Nadano, M. Iwasaki, S. Endo, K. Kitajima, S. Inoue, Y. Inoue, A naturally occurring deaminated neuraminic acid, 3-deoxy-D-glycero-D-galacto-nonulosonic acid (KDN). Its unique occurrence at the nonreducing ends of oligosialyl chains in polysialoglycoprotein of rainbow trout eggs. J. Biol. 261, 11550–11557 (1986)
S. Inoue, S.L. Lin, T. Chang, S.H. Wu, C.W. Yao, T.Y. Chu, Y. Inoue, Identification of free deaminated sialic acid (2-keto-3-deoxy-D-glycero-D-galacto-nononic acid) in human red blood cells and its elevated expression in fetal cord red blood cells and ovarian cancer cells. J. Biol. 273, 27199–27204 (1998). https://doi.org/10.1074/jbc.273.42.27199
S.D. Burke, E.A. Voight, Formal synthesis of (+)-3-Deoxy-D-glycero-D-galacto-2-nonulosonic acid (KDN) via desymmetrization by ring closing metathesis. Org. Lett. 3, 237–240 (2001). https://doi.org/10.1021/ol/0068871
C. Festa, S. De Marino, V. Sepe, M.V. D’Auria, G. Bifulco, C. Débitus, A. Zampella, Solomonamides A and B, new anti-inflammatory peptides from Theonella swinhoei. Org. Lett. 13, 1532–1535 (2011). https://doi.org/10.1021/ol200221n
I. Cheng-Sánchez, C. García-Ruíz, F. Sarabia, An olefin metathesis approach towards the solomonamides. Tetrahedron Lett. 57, 3392–3395 (2016). https://doi.org/10.1016/j.tetlet.2016.06.081
D.L. Wood, L.E. Browne, B. Ewing, K. Lindahl, W.D. Bedard, P.E. Tilden, P.R. Hughes, Western pine beetle: specificity among enantiomers of male and female components of an attractant pheromone. Science 192, 896–898 (1976). https://doi.org/10.1126/science.1273574
J.P. Vité, R.F. Billings, C.W. Ware, K. Mori, Southern pine beetle: enhancement or inhibition of aggregation response mediated by enantiomers of endo-brevicomin. Naturwissenschaften 72, 99–100 (1985). https://doi.org/10.1007/bf00508146
S.D. Burke, N. Muller, C.M. Beaudry, Desymmetrization by ring-closing metathesis leading to 6,8-dioxabicyclo[3.2,1]octanes: A new route for the synthesis of (+)-exo- and endo- brevicomin. Org. Lett. 1, 1827–1829 (1999). https://doi.org/10.1021/ol9910971
J.I. Kobayashi, D. Watanabe, N. Kawasaki, M. Tsuda, Nakadomarin A, a novel hexacyclic manzamine-related alkaloid from Amphimedon sponge. J. Org. Chem. 62, 9236–9239 (1997). https://doi.org/10.1021/jo9715377
M. Tsuda, J.I. Kobayashi, Structures and biogenesis of manzamines and related alkaloids. Heterocycles 46, 765–794 (1997). https://doi.org/10.3987/rev-97-sr5
A. Fürstner, O. Guth, A. Düffels, G. Seidel, M. Liebl, B. Gabor, R. Mynott, Indenylidene complex of ruthenium: optimized synthesis, structure elucidation and performance as catalysts for olefin metathesis-application to the synthesis of the ADE-ring system of nakadomarin A. Chem Eur. J. 7, 4811–4820 (2001). https://doi.org/10.1002/1521-3765(20011119)7:22
K. Sugawara, Y. Nishiyama, S. Toda, N. Komiyama, M. Hatori, T. Moriyama, T. Oki, Lactimidomycin, a new glutarimide group antibiotic. J. Antibiot. 45, 1433–1441 (1992). https://doi.org/10.7164/antibiotics.45.1433
J. Ju, S.R. Rajski, S.K. Lim, J.W. Seo, N.R. Peters, F.M. Hoffmann, B. Shen, Lactimidomycin, iso-migrastatin and related glutarimide-containing 12-membered macrolides are extremely potent inhibitors of cell migration. J. Am. Chem. Soc. 131, 1370–1371 (2009). https://doi.org/10.1021/ja808462p
D. Gallenkamp, A. Fürstner, Stereoselective synthesis of E, Z-configured 1,3-dienes by ring-closing metathesis. application to the total synthesis of lactimidomycine. J. Am. Chem. Soc. 133, 9232–9235 (2011). https://doi.org/10.1021/ja2031085
K. Micoine, A. Fürstner, Concise total synthesis of the potent translation and cell migration inhibitor lactimidomycin. J. Am. Chem. Soc. 132(40), 14064–14066 (2010). https://doi.org/10.1021/ja107141p
S. Samwel, S.J. Mdachi, M.H. Nkunya, B.N. Irungu, M.J. Moshi, B. Moulton, B.S. Luisi, Cleistenolide and cleistodienol: Novel bioactive constituents of Cleistochlamys kirkii. Nat. Prod. Commun. 2, 737–741 (2007). https://doi.org/10.1177/1934578X0700200706
M.H.H. Nkunya, Unusual metabolites from some Tanzanian indigenous plant species. Pure Appl. Chem. 77, 1943–1955 (2005). https://doi.org/10.1351/pac200577111943
D.C. Babu, J.J.P. Selavam, D.K. Reddy, V. Shekhar, Y. Venkateswarlu, Stereoselective total synthesis of (-)-cleistenolide. Tetrahedron 67, 3815–3819 (2011). https://doi.org/10.1016/j.tet.2011.03.107
H. Fukumoto, T. Esumi, J. Ishihara, S. Hatakeyama, Total synthesis of (±)-erythravine based on ring closing dienyne metathesis. Tetrahedron Lett. 44, 8047–8049 (2003). https://doi.org/10.1016/j.tetlet.2003.09.059
G. Sabitha, B. Vangala, S.S.S. Reddy, J. Yadav, Total synthesis of (+)-cryptocaryalactone and a diastereoisomer of (+)-strictifolion via ring-closing metathesis (RCM) and olefin cross-metathesis (CM). Helv. Chim. Acta 93, 329–338 (2010). https://doi.org/10.1002/hlca.200900170
J.S. Yadav, M.R. Kumar, G. Sabitha, Stereoconvergent synthesis of the C1–C11 and C12–C24 fragments of (−)-macrolactin-A. Tetrahedron Lett. 49, 463–466 (2008). https://doi.org/10.1016/j.tetlet.2007.11.107
S.B. Mahato, K.A. Siddiqui, G. Bhattacharya, T. Ghosal, K. Miyahara, M. Sholichin, T. Kawasaki, Structure and stereochemistry of phaseolinic acid: a new acid from Macrophomina phaseolina. J. Nat. Prod. 50, 245–247 (1987). https://doi.org/10.1021/np50050a024
B.K. Park, M. Nakagawa, A. Hirota, M. Nakayama, Methylenolactocin, a novel antitumor antibiotic from Penicillium sp. J. Antibiot. 41, 751–758 (1988). https://doi.org/10.7164/antibiotics.41.751
N. Selvakumar, P.K. Kumar, K.C.S. Reddy, B.C. Chary, Synthesis of substituted butenolides by the ring closing metathesis of two electron deficient olefins: a general route to the natural products of paraconic acids class. Tetrahedron Lett. 48, 2021–2024 (2007). https://doi.org/10.1016/j.tetlet.2007.01.053
I. Jacquemond-Collet, S. Hannedouche, N. Fabre, I. Fourasté, C. Moulis, Two tetrahydroquinoline alkaloids from Galipea officinalis. Phytochemistry 51, 1167–1169 (1999). https://doi.org/10.1016/S0031-9422(99)00032-1
I. Jacquemond-Collet, F. Benoit-Vical, M. Valentin, A. Stanislas, E. Mallié, M. Fourasté, Antiplasmodial and cytotoxic activity of galipinine and other tetrahydroquinolines from Galipea officinalis. Planta Med. 68, 68–69 (2002). https://doi.org/10.1055/s-2002-19869
C. Theeraladanon, M. Arisawa, M. Nakagawa, A. Nishida, Total synthesis of (+)-(S)-angustureine and the determination of the absolute configuration of the natural product angustureine. Tetrahedron Asymm. 16, 827–831 (2005). https://doi.org/10.1016/j.tetasy.2004.12.022
N.R. Guz, P. Lorenz, F.R. Stemitz, New coumarins from Harbouria trachypleura: isolation and synthesis. Tetrahedron Lett. 42, 6491–6494 (2001). https://doi.org/10.1016/S0040-4039(01)01355-7
A. Kucherenko, M.T. Flavin, W.A. Boulanger, A. Khilevich, R.L. Shone, J.D. Rizzo, Z.Q. Xu, Novel approach for synthesis of (±)-calanolide A and its anti-HIV activity. Tetrahedron Lett. 36, 5475–5478 (1995). https://doi.org/10.1016/0040-4039(95)01059-Q
T.N. Van, S. Debenedetti, N.D. Kimpe, Synthesis of coumarins by ring-closing metathesis using Grubbs catalyst. Tetrahedron Lett. 44, 4199–4201 (2003). https://doi.org/10.1016/S0040-4039(03)00902-X
B. Kesteleyn, N. De Kimpe, L. Van Puyvelde, Total synthesis of two naphthoquinone antibiotics, psychorubrin and pentalongin, and their C(1)-substituted alkyl and aryl derivatives. J. Org. Chem. 64, 1173–1179 (1999). https://doi.org/10.1021/jo9811975
B. Kesteleyn, N. De Kimpe, L. Van Puyvelde, Synthesis of two naphthoquinone antibiotics pentalongin and psychorubrin. Synthesis 1999, 1881–1883 (1999). https://doi.org/10.1055/s-1999-3613
S. Claessens, D. Naidoo, D. Mulholland, L. Verschaeve, J. van Staden, N. De Kimpe, Synthesis of pyranonaphthoquinone antibiotics involving the phthalide annulation strategy. Synlett 2006, 0621–0623 (2006). https://doi.org/10.1055/s-2006-926248
T.N. Van, N.D. Kimpe, Synthesis of pyranonaphthoquinone antibiotics involving the ring closing metathesis of avinyl ether. Tetrahedron Lett. 45, 3443–3446 (2004). https://doi.org/10.1016/j.tetlet.2004.03.008
E. Dorta, A.R. Dıaz-Marrero, M. Cueto, L. D’Croz, J.L. Maté, J. Darias, Chamigrenelactone, a polyoxygenated sesquiterpene with a novel structural type and devoid of halogen from Laurencia obtusa. Tetrahedron Lett. 45, 7065–7068 (2004). https://doi.org/10.1016/j.tetlet.2004.07.125
J.D. Martin, C. Perez, J.L. Ravelo, Enantioselective ring construction: Synthesis of halogenated marine natural spiro [5.5] undecane sesquiterpenes. J. Am. Chem Soc. 108, 7801–7811 (1986). https://doi.org/10.1021/ja00284a052
D.E. White, I.C. Stewart, R.H. Grubbs, B.M. Stoltz, The catalytic asymmetric total synthesis of Elatol. J. Am. Chem. Soc. 130, 810–811 (2000). https://doi.org/10.1021/ja710294k
M. Ouedraogo, H. Carreyre, C. Vandebrouck, J. Bescond, G. Raymond, I.P. Guissou, J. Marrot, Structure elucidation of a dihydropyranone from Tapinanthus dodoneifolius. J. Nat. Prod. 70, 2006–2009 (2007). https://doi.org/10.1021/np070355x
S.E. Drewes, B.M. Sehlapelo, M.M. Horn, R. Scott-Shaw, P. Sandor, 5,6-Dihydro-α-pyrones and two bicyclic tetrahydro-α-pyrone derivatives from Cryptocarya latifolia. Phytochemistry 38, 1427–1430 (1995). https://doi.org/10.1016/0031-9422(94)00828-H
B. Chinnababu, S.P. Reddy, C.B. Rao, K. Rajesh, Y. Venkateswarlu, Stereoselective total synthesis of Dodoneine. Helvetica Chem. Acta 93, 1960–1966 (2010). https://doi.org/10.1002/hlca.200900478
T. El-Elimat, H.A. Raja, C.S. Day, W.L. Chen, S.M. Swanson, N.H. Oberlies, Greensporones: resorcylic acid lactones from an aquatic Halenospora sp. J. Nat. Prod. 77, 2088–2098 (2014). https://doi.org/10.1021/np500497r
K. Tadpetch, L. Jeanmard, V. Rukachaisirikul, Total synthesis of greesporone C. Tetrahedron Lett. 58, 3453–3456 (2017). https://doi.org/10.1016/j.tetlet.2017.07.074
G. Lang, M.I. Mitova, G. Ellis, S. van der Sar, R.K. Phipps, J.W. Blunt, M.H. Munro, Bioactivity profiling using HPLC/microtiter-plate analysis: application to a New Zealand marine alga-derived fungus, Gliocladium sp. J. Nat. Prod. 69, 621–624 (2006). https://doi.org/10.1021/np0504917
J.S. Yadav, V. Vardhan, S. Das, Total synthesis of 4-ketoclonostachydiol. Synthesis 46, 2347–2352 (2014). https://doi.org/10.1055/s-0033-1339121
S. Ohta, M.M. Uy, M. Yanai, E. Ohta, T. Hirata, S. Ikegami, Exiguolide, a new macrolide from the marine sponge Geodia exigua. Tetrahedron Lett. 47, 1957–1960 (2006). https://doi.org/10.1016/j.tetlet.2006.01.062
M.S. Kown, S.K. Woo, S.W. Na, E. Lee, Total synthesis of (+)-exiguolide. Angew. Chem. Int. Ed 47, 1733–1735 (2008). https://doi.org/10.1002/anie.200705018
F. Echeverri, V. Arango, W. Quiñones, F. Torres, G. Escobar, Y. Rosero, R. Archbold, Passifloricins, polyketides α-pyrones from Passiflora foetida resin. Phytochemistry 56, 881–885 (2001). https://doi.org/10.1016/S0031-9422(00)00478-7
W. Cardona, W. Quiñones, F. Echeverri, Leishmanicidal activity of passifloricin A and derivatives. Molecules 9, 666–672 (2004). https://doi.org/10.3390/90800666
S. Chandrasekhar, C. Rambabu, A.S. Reddy, Asymmetric synthesis of (+)-passifloricin A and its 6-epimer. Tetrahedron Lett. 49, 4476–4478 (2008). https://doi.org/10.1016/j.tetlet.2008.05.070
D. Stærk, A.K. Lykkeberg, J. Christensen, B.A. Budnik, F. Abe, J.W. Jaroszewski, In vitro cytotoxic activity of phenanthroindolizidine alkaloids from cynanchum v incetoxicum and tylophora t anakae against drug-sensitive and multidrug-resistant cancer cells. J. Nat. Prod. 65, 1299–1302 (2002). https://doi.org/10.1021/np0106384
S. Lebrun, A. Couture, E. Deniau, P. Grandclaudon, Total syntheses of (±)-cryptopleurine, (±)-antofine and (±)-deoxypergularinine. Tetrahedron 55, 2659–2670 (1999)
S. Kim, J. Lee, T. Lee, H.G. Park, D. Kim, First asymmetric total synthesis of (-)-antofine by using an enantioselective catalytic phase transfer alkylation. Org. Lett. 5, 2703–2706 (2003). https://doi.org/10.1021/ol0349007
I. Shiina, H. Fujisawa, T. Ishii, Stereoselective total synthesis of cephalosporolide D. Heterocycles 52, 1105–1123 (2000). https://doi.org/10.3987/com-99-s85
X.P. Fang, J.E. Anderson, X.X. Qiu, J.F. Kozlowski, C.J. Chang, J.L. McLaughlin, Gonioheptolides A and B: Novel eight-membered-ring lactones from Goniothalamus giganteus (Annonaceae). Tetrahedron 49, 1563–1570 (1993). https://doi.org/10.1016/S004-04020(01)80344-6
K.R. Buszek, N. Sato, Y. Jeong, Total synthesis of octalactin A via ring-closing metathesis reaction. Tetrahedron Lett. 43, 181–184 (2002). https://doi.org/10.1016/S0040-4039(01)02078-0
N. Asano, H. Kuroi, K. Ikeda, H. Kiz, Y. Kameda, A. Kato, Fleet GW New polyhydroxylated pyrrolizidine alkaloids from Muscari armeniacum: structural determination and biological activity. Tetrahedron Asymm. 11, 1–8 (2000). https://doi.org/10.1016/S0957-4166(99)00508-X
R.J. Nash, L.E. Fellows, J.V. Dring, G.W. Fleet, A. Girdhar, N.G. Ramsden, A.M. Scofield, Two alexines [3-hydroxymethyl-1,2,7-trihydroxypyrrolizidines] from Castanospermum australe. Phytochemistry 29, 111–114 (1990). https://doi.org/10.1016/0031-9422(90)89022-2
D.L. Taylor, R. Nash, L.E. Fellows, M.S. Kang, A.S. Tyms, Naturally occurring pyrrolizidines: Inhibition of α-glucosidase 1 and anti-HIV activity of one stereoisomer. Antivir Chem Chemother 3, 273–277 (1992). https://doi.org/10.1177/095632029200300504
L. Rambaud, P. Compain, O.R. Martin, First total synthesis of (+)-hyacinthacine A2. Tetrahedron Asymm. 12, 1807–1809 (2001). https://doi.org/10.1016/S0957-4166(01)00324-X
D. Abate, W.R. Abraham, Antimicrobial metabolites from Lentinus crinitus. J. Antibiot. 47, 1348–1350 (1994). https://doi.org/10.7164/antibiotics.47.1348
D.C. Harrowven, M.C. Laucas, P.D. Howes, A total synthesis of (±)-1-Desoxyhypnophilin: using ring closing metathesis for the construction of cyclic enones. Tetrahedron Lett. 41, 8985–8987 (2000). https://doi.org/10.1016/S0040-4039(00)01595-1
N. Oku, K. Takada, R.W. Fuller, J.A. Wilson, M.L. Peach, L.K. Pannell, K.R. Gustafson, Isolation, structural elucidation, and absolute stereochemistry of enigmazole A, a cytotoxic phosphomacrolide from the Papua New Guinea marine sponge Cinachyrella enigmatica. J. Am. Chem. Soc. 132, 10278–10285 (2010). https://doi.org/10.1021/ja1016766
S. Hirota, K. Isozaki, Y. Moriyama, K. Hashimoto, T. Nishida, S. Ishiguro, G.M. Tunio, Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 279, 577–580 (1998). https://doi.org/10.1126/science.279.5350.577
K. Sakurai, M. Sasaki, H. Fuwa, Total synthesis of (-)-enigmazole A. Angew. Chem. Int. Ed. 57, 5143–5146 (2018). https://doi.org/10.1002/anie.201801561
T.C. McMorris, R. Lira, P.K. Gantzel, M.J. Kelner, R. Dawe, Sesquiterpenes from the Basidiomycete Omphalotus i lludens. J. Nat. Prod. 63, 1557–1559 (2000). https://doi.org/10.1021/np9904760
G. Liu, D. Romo, Total synthesis of (+)-omphadiol. Angew. Chem. Int. Ed. 50, 7537–7540 (2011). https://doi.org/10.1002/anie.201102289
M.I. Aguilar, F. Giral, O. Espejo, Alkaloids from the flowers of Erythrina americana. Phytochemistry 20, 2061–2062 (1981). https://doi.org/10.1016/0031-9422(81)84079-4
H. Fukumoto, K. Takahashi, J. Ishihara, S. Hatakeyama, Total synthesis of (+)-β-erythroidine. Angew. Chem. Int. Ed. 45, 2731–2134 (2006). https://doi.org/10.1002/anie.2006002109
U. Renner, P. Kernweisz, Alkaloide ausSchizozygia caffaeoides (Boj.) Baill. Experientia 19, 244–246 (1963). https://doi.org/10.1007/BF02151358
Y. Miura, N. Hayashi, S. Yokoshima, Total synthesis of (-)-isoschizogamine. J. Am. Chem. Soc. 134, 11995–11997 (2012). https://doi.org/10.1021/ja305856q
S. Kobayashi, K. Tsuchiya, T. Harada, M. Nishide, T. Kurokawa, T. Nakagawa, K. Kobayashi, Pironetin, a novel plant growth regulator produced by Streptomyces sp. NK10958. J. Antibiot. 47, 697–702 (1994). https://doi.org/10.7164/antibiotics.49.173
C. Bressy, J.P. Vors, S. Hillebrand, S. Arseniyadis, J. Cossy, Asymmetric total synthesis of the immunosuppressant (-)-pironetin. Angew. Chem. Int. Ed. 47, 10137–10140 (2008). https://doi.org/10.1002/anie.200802423
D.G. Corley, M.S. Tempesta, M.M. Iwu, Convulsant alkaloids from Dioscorea dumetorum. Tetrahedron Lett. 26, 1615–1618 (1985)
A. Rückert, P.H. Deshmukh, S. Blechert, Catalytic enantioselective total synthesis of (+)-dumetorine by ring-rearrangement metathesis. Tetrahedron Lett. 47, 7977–7981 (2006). https://doi.org/10.1016/j.tetlet.2006.08.114
M. Dochnahl, S.R. Schulz, S. Blechert, Enantioselective total synthesis of (-)-trans-Dendrochrysine via a ring- rearrangement metathesis approach. Synlett. 16, 2599–2601 (2007). https://doi.org/10.1055/s-2007-986672
S. Mill, C. Hootelé, Alkaloids of Andrachne aspera. J. Nat. Prod. 63, 762–764 (2000). https://doi.org/10.1021/np9905214
P.R. Krishna, G. Dayaker, A stereoselective total synthesis of (-)-andrachcinidine via an olefin metathesis protocol. Tetrahedron Lett. 48, 7279–7282 (2007). https://doi.org/10.1016/j.tetlet.2007.08.053
K. Kito, R. Ookura, S. Yoshida, M. Namikoshi, T. Ooi, T. Kusumi, New cytotoxic 14-membered macrolides from marine-derived fungus Aspergillus ostianus. Org. Lett. 10, 225–228 (2008). https://doi.org/10.1021/ol702598q
S. Díaz-Oltra, C.A. Angulo-Pachón, J. Murga, M. Carda, J.A. Marco, Stereoselective synthesis of the cytotoxic 14-membered macrolide aspergillide A. J. Org. Chem. 75, 1775–1778 (2010). https://doi.org/10.1021/jo9027038
B.R. Kammari, N.K. Bejjanki, N. Kommu, Total synthesis of decytospolides A, B and a formal synthesis of Aspergillide A starting from D-mannitol via tandem/domino reactions by Grubb’s catalysts. Tetrahedron Asymm. 26, 296–303 (2015). https://doi.org/10.1016/j.tetasy.2015.01.014
A.J. Cavalheiro, M. Yoshida, 6-[ω-arylalkenyl]-5, 6-dihydro-α-pyrones from Cryptocarya moschata (Lauraceae). Phytochemistry 53, 811–819 (2000). https://doi.org/10.1016/s0031942299005324
S. Zschocke, J. Van Staden, Cryptocarya species-substitute plants for Ocotea bullata A pharmacological investigation in terms of cyclooxygenase-1 and-2 inhibition. J. Ethnopharmacol. 71, 473–478 (2000). https://doi.org/10.1016/S0378874100001835
G. Sabita, S.S.S. Reddy, J.S. Yaday, Stereoselective total synthesis of cryptopyranmoscatone A1. Tetrahedron Lett. 52, 2407–2409 (2011). https://doi.org/10.1016/j.tetlet.2011.02.107
A. Hisham, M. Toubi, W. Shuaily, M.A. Bai, Y. Fujimoto, Cardiobutanolide, a styryl lactone from Goniothalamus cardiopetalus. Phytochemistry 62, 597–600 (2003). https://doi.org/10.1016/s0031-94220200536-8
M.C. Zafra-Polo, B. Figadère, T. Gallardo, J. Tormo, D. Cortes, Natural acetogenins, synthesis and mechanisms of action. Phytochemistry 48, 1087–1117 (1998). https://doi.org/10.1016/s0031-94229700917-5
P.R. Krishna, E.S. Kumar, Olefin cross-metathesis based approach for stereoselective total synthesis of (+)-cardiobutanolide. Tetrahedron Lett. 50, 6676–6679 (2009). https://doi.org/10.1016/j.tetlet.2009.09.077
S. Kadota, J.K. Prasain, J.X. Li, P. Basnet, H. Dong, T. Tani, T. Namba, Blepharocalyxins A and B, novel diarylheptanoids from Alpinia blepharocalyx, and their inhibitory effect on NO formation in murine macrophages. Tetrahedron Lett. 37, 7283–7286 (1996). https://doi.org/10.1016/0040-40399601649-8
H.M. Ko, D.G. Lee, M.A. Kim, H.J. Kim, J. Park, M.S. Lah, E. Lee, Total synthesis of (-)-Blepharocalyxin D. Org. Lett. 9, 141–144 (2007). https://doi.org/10.1021/ol0627956
A. Cutignano, G. Nuzzo, D. D’Angelo, E. Borbone, A. Fusco, A. Fontana, Mycalol: a natural lipid with promising cytotoxic properties against human anaplastic thyroid carcinoma cells. Ang. Chem. 125, 9426–9430 (2013). https://doi.org/10.1002/ange.201303039
B. Seetharamsingh, P.R. Rajamohanan, D.S. Reddy, Total synthesis and structural revision of Mycalol, an anticancer natural product from the marine source. Org. Lett. 17, 1652–1655 (2015). https://doi.org/10.1021/acs.orglett.5b00345
Acknowledgements
The authors are thankful to GC University, Faisalabad, for providing the facilities to carry out this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bano, T., Zahoor, A.F., Rasool, N. et al. Recent trends in Grubbs catalysis toward the synthesis of natural products: a review. J IRAN CHEM SOC 19, 2131–2170 (2022). https://doi.org/10.1007/s13738-021-02463-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-021-02463-x