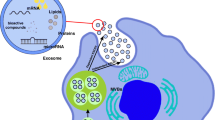
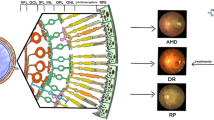
Abstract
As an important part of the central nervous system (CNS), the optic nerve usually cannot regenerate directly after injury. Therefore, treating the injury and restoring the function of the optic nerve are a historical problem in the medical field. Due to the special anatomical position of the optic nerve, the microenvironment needed for protection and regeneration after injury is lacking. Therefore, preventing the continued loss of neurons, protecting the functional nerves, and promoting the effective protection of nerves are the main ways to solve the problem. Exosomes are nano-sized vesicles with a diameter of 30–150 nm, composed of lipid bilayers, proteins, and genetic material. They have key functions in cell-to-cell communication, immune regulation, inflammation, and regeneration. More and more shreds of evidence show that exosomes not only play an important role in systemic diseases such as cancer, cardiovascular diseases, and brain diseases; they also play a key role in ophthalmological diseases. This article reviews the role of exosomes in the protection and regeneration of the optic nerve after optic nerve injury in related experimental studies and clinical treatment methods.
Graphical abstarct

.
Similar content being viewed by others
Availability of data and materials
Not applicable.
Abbreviations
- Ago2:
-
Argonaute 2
- ATG3:
-
Autophagy-related 3
- BNIP3:
-
Bcl-2-interacting protein 3
- BTC:
-
Betacellulin
- CNS:
-
Central nervous system
- DAB2IP:
-
Disabled homolog 2-interacting protein
- ESEs:
-
Early sorted inclusion bodies
- EVs:
-
Extracellular vesicles
- EXOPACAP38:
-
PACAP38 is loaded by exosomal anchoring peptide CP05
- Frs2:
-
Fibroblast growth factor receptor substrate 2
- GAP43:
-
Growth associated protein-43
- GLT-1:
-
Glutamate transporter-1
- GSK3b:
-
Glycogen synthase kinase 3 beta
- IOP:
-
Intraocular pressure
- LSE:
-
Late classified inclusion bodies
- MNU:
-
Methyl-N-nitrosourea
- MSC:
-
Mesenchymal stromal cells
- mTOR:
-
Mammalian target of rapamycin
- MVBs:
-
Multivesicular bodies
- Pdcd4:
-
Programmed cell death 4
- PI3K:
-
Phosphatidylinositol 3-kinase
- PTEN:
-
Phosphatase and tensin homolog deleted on chromosome ten
- RGC:
-
Retinal ganglion cell
- RVG:
-
Exoglycoprotein
- TON:
-
Traumatic optic neuropathy
- TSC2:
-
Tuberous sclerosis 2
References
Schumer R, Podos S. The nerve of glaucoma! Archiv Ophthalmol (Chicago, Ill: 1960). 1994;112(1):37–44.
Lewin GR, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci. 1996;19:289–317.
Laha B, Stafford BK, Huberman AD. Regenerating optic pathways from the eye to the brain. Science. 2017;356(6342):1031–4.
Fruhbeis C, Frohlich D, Kuo WP, Kramer-Albers EM. Extracellular vesicles as mediators of neuron-glia communication. Front Cell Neurosci. 2013;7:182.
Alenquer M, Amorim MJ. Exosome biogenesis, regulation, and function in viral infection. Viruses. 2015;7(9):5066–83.
Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. 2019;8:307.
Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262:9412–20.
Hajrasouliha AR, Jiang G, Lu Q, Lu H, Kaplan HJ, Zhang HG, et al. Exosomes from retinal astrocytes contain antiangiogenic components that inhibit laser-induced choroidal neovascularization. J Biol Chem. 2013;288(39):28058–67.
Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stromal cells. Int J Mol Sci. 2014;15:4142–57.
Vlassov A, Magdaleno S, Setterquist R, Conrad R. Exosomes current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta Gen Subj. 2012;1820:940–8.
Zöller M. Tetraspanins: push and pull in suppressing and promoting metastasis. Nat Rev Cancer. 2009;9:40–55.
Castro-Manrreza ME, Montesinos JJ. Immunoregulation by mesenchymal stromal cells: biological aspects and clinical applications. J Immunol Res. 2015;2015:394917.
Mishra VK, Shih HH, Parveen F, Lenzen D, Ito E, Chan TF, et al. Identifying the therapeutic significance of mesenchymal stem cells. Cells. 2020;9(5):1145.
Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403.
Yu B, Zhang X, Li X. Exosomes are derived from mesenchymal stem cells. Int J Mol Sci. 2014;15:4142–57.
Mesentier-Louro LA, Zaverucha-do-Valle C, Rosado-de-Castro PH, Silva-Junior AJ, Pimentel-Coelho PM, Mendez-Otero R, et al. Bone marrow-derived cells as a therapeutic approach to optic nerve diseases. Stem Cells Int. 2016;2016:5078619.
Nuzzi R, Tridico F. Perspectives of autologous mesenchymal stem-cell transplantation in macular hole surgery: a review of current findings. J Ophthalmol. 2019;2019:8.
Nuzzi R, Buschini E. Prospettive di terapia oculare. Miner Oftalmol. 2010;52(2):55–61.
Xuqian W, Kanghua L, WeiHong Y. Intraocular transplantation of human adipose-derived mesenchymal stromal cells in a rabbit model of experimental retinal holes. Ophthal Res. 2011;46:199–207.
da Silva-Junior AJ, Mesentier-Louro LA, Nascimento-Dos-Santos G, Teixeira-Pinheiro LC, Vasques JF, Chimeli-Ormonde L, et al. Human mesenchymal stem cell therapy promotes retinal ganglion cell survival and target reconnection after optic nerve crush in adult rats. Stem Cell Res Ther. 2021;12(1):69.
Chung S, Rho S, Kim G, Kim SR, Baek KH, Kang M, et al. Human umbilical cord blood mononuclear cells and chorionic plate-derived mesenchymal stromal cells promote axon survival in a rat model of optic nerve crush injury. Int J Mol Med. 2016;37(5):1170–80.
Yeo RWY, Lai RC, Zhang B. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev. 2003;65:336–41.
Sze SK, de Kleijn DP, Lai RC, Khia Way Tan E, Zhao H, Yeo KS, et al. Elucidating the secretion proteome of human embryonic stem cell-derived mesenchymal stromal cells. Mol Cell Proteomics. 2007;6(10):1680–9.
Nakano T, Ando S, Takata N, Kawada M, Muguruma K, Sekiguchi K, et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 2012;10(6):771–85.
Wooff Y, Cioanca AV, Chu-Tan JA, Aggio-Bruce R, Schumann U, Natoli R. Small-medium extracellular vesicles and their miRNA cargo in retinal health and degeneration: mediators of homeostasis, and vehicles for targeted gene therapy. Front Cell Neurosci. 2020;14:160.
Harrell CR, Fellabaum C, Arsenijevic A, Markovic BS, Djonov V, Volarevic V. Therapeutic potential of mesenchymal stromal cells and their secretome in the treatment of glaucoma. Stem Cells Int. 2019;2019:7869130.
Yang J, Zhang X, Chen X, Wang L, Yang G. Exosome mediated delivery of miR-124 promotes neurogenesis after ischemia. Mol Ther Nucleic Acids. 2017;7:278–87.
Katsuda T, Ochiya T. Molecular signatures of mesenchymal stem cell-derived extracellular vesicle-mediated tissue repair. Stem Cell Res Ther. 2015;6:212.
Wang T, Li Y, Guo M, Dong X, Liao M, Du M, et al. Exosome-mediated delivery of the neuroprotective peptide PACAP38 promotes retinal ganglion cell survival and axon regeneration in rats with traumatic optic neuropathy. Front Cell Dev Biol. 2021;9:659783.
Mead B, Amaral J, Tomarev S. Mesenchymal stem cell-derived small extracellular vesicles promote neuroprotection in rodent models of glaucoma. Investig Ophthalmol Vis Sci. 2018;59(2):702–14.
Li R, Jin Y, Li Q, Sun X, Zhu H, Cui H. MiR-93-5p targeting PTEN regulates the NMDA-induced autophagy of retinal ganglion cells via AKT/mTOR pathway in glaucoma. Biomed Pharmacother. 2018;100:1–7.
Mak HK, Yung JSY, Weinreb RN, Ng SH, Cao X, Ho TYC, et al. MicroRNA-19a-PTEN axis is involved in the developmental decline of axon regenerative capacity in retinal ganglion cells. Mol Ther Nucleic Acids. 2020;21:251–63.
Marler KJ, Suetterlin P, Dopplapudi A, Rubikaite A, Adnan J, Maiorano NA, et al. BDNF promotes axon branching of retinal ganglion cells via miRNA-132 and p250GAP. J Neurosci. 2014;34(3):969–79.
Zhang QL, Wang W, Li J, Tian SY, Zhang TZ. Decreased miR-187 induces retinal ganglion cell apoptosis through upregulating SMAD7 in glaucoma. Biomed Pharmacother. 2015;75:19–25.
Zhang Q, He C, Li R, Ke Y, Sun K, Wang J. miR-708 and miR-335-3p inhibit the apoptosis of retinal ganglion cells through suppressing autophagy. J Mol Neurosci. 2021;71(2):284–92.
Nie XG, Fan DS, Huang YX, He YY, Dong BL, Gao F. Downregulation of microRNA-149 in retinal ganglion cells suppresses apoptosis through activation of the PI3K/Akt signaling pathway in mice with glaucoma. Am J Physiol Cell Physiol. 2018;315(6):C839–49.
Yang J, Wang N, Luo X. Intraocular miR-211 exacerbates pressure-induced cell death in retinal ganglion cells via direct repression of FRS2 signaling. Biochem Biophys Res Commun. 2018;503(4):2984–92.
Moisseiev E, Anderson JD, Oltjen S, Goswami M, Zawadzki RJ, Nolta JA, et al. Protective effect of intravitreal administration of exosomes derived from mesenchymal stromal cells on retinal ischemia. Curr Eye Res. 2017;42(10):1358–67.
Yuan FY, Zhang MX, Shi YH, Li MH, Ou JY, Bai WF, et al. Bone marrow stromal cells-derived exosomes target DAB2IP to induce microglial cell autophagy, a new strategy for neural stem cell transplantation in brain injury. Exp Ther Med. 2020;20(3):2752–64.
Li X, Wang Q, Ren Y, Wang X, Cheng H, Yang H, et al. Tetramethylpyrazine protects retinal ganglion cells against H2O2induced damage via the microRNA182/mitochondrial pathway. Int J Mol Med. 2019;44(2):503–12.
Deng CL, Hu CB, Ling ST, Zhao N, Bao LH, Zhou F, et al. Photoreceptor protection by mesenchymal stem cell transplantation identifies exosomal MiR-21 as a therapeutic for retinal degeneration. Cell Death Differ. 2021;28(3):1041–61.
Cong M, Shen M, Wu X, Li Y, Wang L, He Q, et al. Improvement of sensory neuron growth and survival via negatively regulating PTEN by miR-21-5p-contained small extracellular vesicles from skin precursor-derived Schwann cells. Stem Cell Res Ther. 2021;12(1):80.
Men Y, Yelick J, Jin S, Tian Y, Chiang MSR, Higashimori H, et al. Exosome reporter mice reveal the involvement of exosomes in mediating neuron to astroglia communication in the CNS. Nat Commun. 2019;10(1):4136.
de Lima S, Koriyama Y, Kurimoto T, Oliveira JT, Yin Y, Li Y, et al. Full-length axon regeneration in the adult mouse optic nerve and partial recovery of simple visual behaviors. Proc Natl Acad Sci USA. 2012;109(23):9149–54.
Tassew N, Charish J, Shabanzadeh A, Luga V, Harada H, Farhani N, et al. Exosomes mediate mobilization of autocrine wnt10b to promote axonal regeneration in the injured CNS. Cell Rep. 2017;20(1):99–111.
Koh K, Park M, Bae ES, Duong VA, Park JM, Lee H, et al. UBA2 activates Wnt/beta-catenin signaling pathway during the protection of R28 retinal precursor cells from hypoxia by extracellular vesicles derived from placental mesenchymal stromal cells. Stem Cell Res Ther. 2020;11(1):428.
Ortega A, Martinez-Arroyo O, Forner MJ, Cortes R. Exosomes as drug delivery systems: endogenous nanovehicles for treatment of systemic lupus erythematosus. Pharmaceutics. 2020;13(1):3.
Mead B, Ahmed Z, Tomarev S. Mesenchymal stem cell-derived small extracellular vesicles promote neuroprotection in a genetic DBA/2J mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2018;59:5473–80.
Mead B, Amaral J, Tomarev S. Mesenchymal stem cell-derived small extracellular vesicles promote neuroprotection in rodent models of glaucoma. Investig Ophthalmol Vis Sci. 2018;59:702–14.
Dong X, Lei Y, Yu Z, Wang T, Liu Y, Han G, et al. Exosome-mediated delivery of an anti-angiogenic peptide inhibits pathological retinal angiogenesis. Theranostics. 2021;11(11):5107–26.
Yonar M, Uehara M, Banouni N, Kasinath V, Li X, Jiang L, et al. Cellular mechanisms of rejection of optic and sciatic nerve transplants: an observational study. Transplant Direct. 2020;6(8):e589.
Suzuki N, Shimizu J, Takai K, Arimitsu N, Ueda Y, Takada E, et al. Establishment of retinal progenitor cell clones by transfection with Pax6 gene of mouse induced pluripotent stem (iPS) cells. Neurosci Lett. 2012;509(2):116–20.
Gokoffski KK, Lam P, Alas BF, Peng MG, Ansorge HRR. Optic nerve regeneration: how will we get there? J Neuroophthalmol. 2020;40(2):234–42.
Funding
This work was supported by the Natural Science Grant of the Heilongjiang province of China (H2018035, No. 2020H040), and The Innovation and Development Foundation of First Affiliated Hospital of Harbin Medical University (2018L002).
Author information
Authors and Affiliations
Contributions
HZL carried out literature searches, and did data interpretation and writing; HZL did writing and editing; FW, YS, and FT conceptualized the paper, and did writing and editing. The authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors have no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, H., Su, Y., Wang, F. et al. Exosomes: a new way of protecting and regenerating optic nerve after injury. Human Cell 35, 771–778 (2022). https://doi.org/10.1007/s13577-022-00688-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-022-00688-3