Abstract
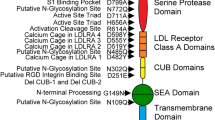
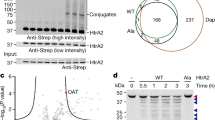
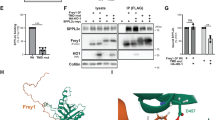
The integral membrane, Kunitz-type, serine protease inhibitors, HAI-1 and HAI-2, closely resemble one another structurally and with regard to their specificity and potency against proteases. Structural complementarity between the Kunitz domains and serine protease domains renders the membrane-associated serine proteases, matriptase and prostasin, the primary target proteases of the HAIs. The shared biochemical enzyme–inhibitor relationships are, however, at odds with their behavior at the cellular level, where HAI-1 appears to be the default inhibitor of these proteases and HAI-2 a cell-type-selective inhibitor, even though they are widely co-expressed. The limited motility of these proteins caused by their membrane anchorages may require their co-localization within a certain distance to allow the establishment of a cellular level functional relationship between the proteases and the inhibitors. The differences in their subcellular localization with HAI-1 both inside the cell and on the cell surface, compared to HAI-2 predominately in intracellular granules has, therefore, been implicated in the differential manner of their control of matriptase and prostasin proteolysis. The targeting signals present in the intracellular domains of the HAIs are systematically investigated herein. Studies involving domain swap and point mutation, in combination with immunocytochemistry and cell surface biotinylation/avidin depletion, reveal that the different subcellular localization between the HAIs can largely be attributed to differences in the intracellular Arg/Lys-rich and EHLVY motifs. These intrinsic differences in the targeting signal render the HAIs as two independent rather than redundant proteolysis regulators.









Similar content being viewed by others
References
Lin CY, Wang JK, Johnson MD. The spatiotemporal control of human matriptase action on its physiological substrates: a case against a direct role for matriptase proteolytic activity in profilaggrin processing and desquamation. Hum Cell. 2020;33:459–69.
Shimomura T, Denda K, Kitamura A, Kawaguchi T, Kito M, Kondo J, et al. Hepatocyte growth factor activator inhibitor, a novel Kunitz-type serine protease inhibitor. J Biol Chem. 1997;272:6370–6.
Kawaguchi T, Qin L, Shimomura T, Kondo J, Matsumoto K, Denda K, et al. Purification and cloning of hepatocyte growth factor activator inhibitor type 2, a Kunitz-type serine protease inhibitor. J Biol Chem. 1997;272:27558–64.
Lin CY, Anders J, Johnson M, Dickson RB. Purification and characterization of a complex containing matriptase and a Kunitz-type serine protease inhibitor from human milk. J Biol Chem. 1999;274:18237–42.
Lai CH, Lai YJJ, Chou FP, Chang HHD, Tseng CC, Johnson MD, et al. Matriptase complexes and prostasin complexes with HAI-1 and HAI-2 in human milk: Significant proteolysis in lactation. PLoS ONE. 2016;33:11.
Oberst MD, Williams CA, Dickson RB, Johnson MD, Lin CY. The activation of matriptase requires its noncatalytic domains, serine protease domain, and its cognate inhibitor. J Biol Chem. 2003;278:26773–9.
Lee MS, Tseng IC, Wang Y, Kiyomiya KI, Johnson MD, Dickson RB, et al. Autoactivation of matriptase in vitro: Requirement for biomembrane and LDL receptor domain. Am J Physiol - Cell Physiol. 2007;293:C95.
Tseng IC, Xu H, Chou FP, Li G, Vazzano AP, Kao JPY, et al. Matriptase activation, an early cellular response to acidosis. J Biol Chem. 2010;285:3261–70.
List K, Szabo R, Molinolo A, Sriuranpong V, Redeye V, Murdock T, et al. Deregulated matriptase causes ras-independent multistage carcinogenesis and promotes ras-mediated malignant transformation. Genes Dev. 2005;19:1934–50.
Szabo R, Molinolo A, List K, Bugge TH. Matriptase inhibition by hepatocyte growth factor activator inhibitor-1 is essential for placental development. Oncogene. 2007;26:1546–56.
Szabo R, Hobson JP, List K, Molinolo A, Lin CY, Bugge TH. Potent inhibition and global co-localization implicate the transmembrane Kunitz-type serine protease inhibitor hepatocyte growth factor activator inhibitor-2 in the regulation of epithelial matriptase activity. J Biol Chem. 2008;283:29495–504.
Szabo R, Kosa P, List K, Bugge TH. Loss of matriptase suppression underlies Spint1 mutation-associated ichthyosis and postnatal lethality. Am J Pathol. 2009;174:2015–22.
Szabo R, Uzzun Sales K, Kosa P, Shylo NA, Godiksen S, Hansen KK, et al. Reduced Prostasin (CAP1/PRSS8) Activity Eliminates HAI-1 and HAI-2 Deficiency-Associated Developmental Defects by Preventing Matriptase Activation. PLoS Genet. 2012;8:e1002937.
Nonboe AW, Krigslund O, Soendergaard C, Skovbjerg S, Friis S, Andersen MN, et al. HAI-2 stabilizes, inhibits and regulates SEA-cleavage-dependent secretory transport of matriptase. Traffic. 2017;18:378–91.
Szabo R, Bugge TH. Loss of HAI-2 in mice with decreased prostasin activity leads to an early-onset intestinal failure resembling congenital tufting enteropathy. PLoS ONE. 2018;13:660.
Lai YJJ, Chang HHD, Lai H, Xu Y, Shiao F, Huang N, et al. N-glycan branching affects the subcellular distribution of and inhibition of matriptase by HAI-2/placental bikunin. PLoS One. 2015;10.
Laskowski M, Kato I. Protein inhibitors of proteinases. Annu Rev Biochem. 1980;49:593–626.
Chen YW, Wang JK, Chou FP, Chen CY, Rorke EA, Chen LM, et al. Regulation of the matriptase-prostasin cell surface proteolytic cascade by hepatocyte growth factor activator inhibitor-1 during epidermal differentiation. J Biol Chem. 2010;285:31755–62.
Shiao F, Liu LCO, Huang N, Lai YJJ, Barndt RJ, Tseng CC, et al. Selective inhibition of prostasin in human enterocytes by the integral membrane kunitz-type serine protease inhibitor HAI-2. PLoS One. 2017;12.
Chiu YL, Wu YY, Barndt RB, Yeo YH, Lin YW, Sytwo HP, et al. Aberrant regulation favours matriptase proteolysis in neoplastic B-cells that co-express HAI-2. J Enzyme Inhib Med Chem. 2019;34:692–702.
Chang HHD, Xu Y, Lai H, Yang X, Tseng CC, Lai YJJ, et al. Differential subcellular localization renders HAI-2 a matriptase inhibitor in breast cancer cells but not in mammary epithelial cells. PLoS One. 2015;10.
Heinz-Erian P, Müller T, Krabichler B, Schranz M, Becker C, Rüschendorf F, et al. Mutations in SPINT2 cause a syndromic form of congenital sodium diarrhea. Am J Hum Genet. 2008;84:188–96.
Szabo R, Hobson JP, Christoph K, Kosa P, List K, Bugge TH. Regulation of cell surface protease matriptase by HAI2 is essential for placental development, neural tube closure and embryonic survival in mice. Development. 2009;136:2653–63.
Carney TJ, von der Hardt S, Sonntag C, Amsterdam A, Topczewski J, Hopkins N, et al. Inactivation of serine protease Matriptase1a by its inhibitor Hai1 is required for epithelial integrity of the zebrafish epidermis. Development. 2007;134:3461–71.
Su HC, Liang YA, Lai YJJ, Chiu YL, Barndt RB, Shiao F, et al. Natural endogenous human matriptase and prostasin undergo zymogen activation via independent mechanisms in an uncoupled manner. PLoS One. 2016;11.
Lee SP, Kao CY, Chang SC, Chiu YL, Chen YJ, Chen MHG, et al. Tissue distribution and subcellular localizations determine in vivo functional relationship among prostasin, matriptase, HAI-1, and HAI-2 in human skin. PLoS One. 2018;13.
Chiu YL, Wu YY, Barndt RB, Lin YW, Sytwo HP, Cheng A, et al. Differential subcellular distribution renders HAI-2 a less effective protease inhibitor than HAI-1 in the control of extracellular matriptase proteolytic activity. Genes Dis. 2021;
Kiyomiya KI, Lee MS, Tseng IC, Zuo H, Barndt RJ, Johnson MD, et al. Matriptase activation and shedding with HAI-1 is induced by steroid sex hormones in human prostate cancer cells, but not in breast cancer cells. Am J Physiol - Cell Physiol. 2006;291:40–9.
Tseng CC, Jia B, Barndt R, Gu Y, Chen CY, Tseng IC, et al. Matriptase shedding is closely coupled with matriptase zymogen activation and requires de novo proteolytic cleavage likely involving its own activity. PLoS One. 2017;12.
Oberst MD, Chen LYL, Kiyomiya KI, Williams CA, Lee MS, Johnson MD, et al. HAI-1 regulates activation and expression of matriptase, a membrane-bound serine protease. Am J Physiol - Cell Physiol. 2005;289:C462–70.
Wu BY, Lee SP, Hsiao HC, Chiu H, Chen CY, Yeo YH, et al. Matriptase expression and zymogen activation in human pilosebaceous unit. J Histochem Cytochem. 2014;62:50–9.
Chen YW, Wang JK, Chou FP, Wu BY, Hsiao HC, Chiu H, et al. Matriptase regulates proliferation and early, but not terminal, differentiation of human keratinocytes. J Invest Dermatol. 2014;134:405–14.
Lai CH, Chang SC, Chen YJ, Wang YJJ, Lai YJJ, Chang HHD, et al. Matriptase and prostasin are expressed in human skin in an inverse trend over the course of differentiation and are targeted to different regions of the plasma membrane. Biol Open. 2016;5:1380–7.
Chang SC, Chiang CP, Lai CH, Du PWA, Hung YS, Chen YH, et al. Matriptase and prostasin proteolytic activities are differentially regulated in normal and wounded skin. Hum Cell. 2020;33:990–1005.
Kataoka H, Itoh H, Uchino H, Hamasuna R, Kitamura N, Nabeshima K, et al. Conserved expression of hepatocyte growth factor activator inhibitor type-2/placental bikunin in human colorectal carcinomas. Cancer Lett. 2000;148:127–34.
Barndt RB, Lee M-J, Huang N, Lu DD, Lee S-C, Du P-W, et al. Targeted HAI-2 deletion causes excessive proteolysis with prolonged active prostasin and depletion of HAI-1 monomer in intestinal but not epidermal epithelial cells. Hum Mol Genet. https://doi.org/10.1093/hmg/ddab150/6292183
Borgese N. Getting membrane proteins on and off the shuttle bus between the endoplasmic reticulum and the Golgi complex. J Cell Sci. 2016;129:1537–45.
Guo Y, Sirkis DW, Schekman R. Protein sorting at the trans-Golgi network. Annu Rev Cell Dev Biol. 2014;30:169–206.
Nilsson T, Jackson M, Peterson PA. Short cytoplasmic sequences serve as retention signals for transmembrane proteins in the endoplasmic reticulum. Cell. 1989;58:707–18.
Schutze MP, Peterson PA, Jackson MR. An N-terminal double-arginine motif maintains type II membrane proteins in the endoplasmic reticulum. EMBO J. 1994;13:1696–705.
Hawkins LM, Prybylowski K, Chang K, Moussan C, Stephenson FA, Wenthold RJ. Export from the endoplasmic reticulum of assembled N-methyl-D-aspartic acid receptors is controlled by a motif in the C terminus of the NR2 subunit. J Biol Chem. 2004;279:28903–10.
Yang W, Zheng C, Song Q, Yang X, Qiu S, Liu C, et al. A three amino acid tail following the TM4 region of the N-methyl-D-aspartate receptor (NR) 2 subunits is sufficient to overcome endoplasmic reticulum retention of NR1-1a subunit. J Biol Chem. 2007;282:9269–78.
Acknowledgements
This study was supported by National Cancer Institute (NCI) Grant R01 CA 123223 (to MDJ and CYL), Grant (MND-MAB-110-045) from the Ministry of National Defense Medical Affairs Bureau, Taiwan (to JKW), and Grants (CMNDMC10807; CMNDMC10905) from Chi-Mei Medical Center, Tainan, Taiwan (to S-M Huang). We also acknowledge the assistance provided by the Microscopy and Imaging Shared Resource and the Tissue Culture Shared Resource, which are supported in part by the Lombardi Comprehensive Cancer Center support grant (NIH/NCI grant P30-CA051008). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There is no conflict of interest among the authors of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huang, N., Barndt, R.B., Lu, D.D. et al. The difference in the intracellular Arg/Lys-rich and EHLVY motifs contributes to distinct subcellular distribution of HAI-1 versus HAI-2. Human Cell 35, 163–178 (2022). https://doi.org/10.1007/s13577-021-00632-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-021-00632-x