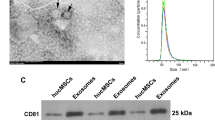
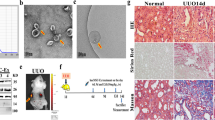
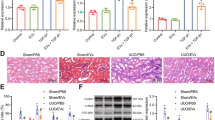
Abstract
Evidence has shown that mesenchymal stem cells’ (MSCs) therapy has potential application in treating chronic kidney disease (CKD). In addition, MSCs-derived exosomes can improve the renal function and prevent the progression of CKD. However, the mechanisms by which MSCs-derived exosomes (MSCs-Exo) ameliorate renal fibrosis in CKD remain largely unclear. To mimic an in vitro model of renal fibrosis, rat kidney tubular epithelial cells (NRK52E) were stimulated with transforming growth factor (TGF)-β1. In addition, we established an in vivo model of unilateral ureteric obstruction (UUO)-induced renal fibrosis. Meanwhile, we exploited exosomes derived from MSCs for delivering miR-186-5p agomir into NRK52E cells or kidneys in vitro and in vivo. In this study, we found that level of miR-186-5p was significantly downregulated in TGF-β1-stimulated NRK52E cells and the obstructed kidneys of UUO mice. In addition, miR-186-5p can be transferred from MSCs to NRK52E cells via exosomes. MSCs-delivered miR-186-5p markedly reduced the accumulation of extracellular matrix (ECM) protein, and inhibited epithelial-to-mesenchymal transition (EMT) and apoptosis in TGF-β1-stimulated NRK52E cells. Moreover, exosomal miR-186-5p from MSCs attenuated kidney injury and fibrosis in a UUO mouse model via inhibition of the ECM protein accumulation and EMT process. Meanwhile, dual-luciferase assay showed that miR-186-5p downregulated Smad5 expression via direct binding with the 3′-UTR of Smad5. Collectively then, these findings indicated that exosomal miR-186-5p derived from MSCs could attenuate renal fibrosis in vitro and in vivo by downregulation of Smad5. These findings may help to understand the role of MSCs’ exosomes in alleviating renal fibrosis in CKD.









Similar content being viewed by others
References
Schieppati A, Remuzzi G. Chronic renal diseases as a public health problem: epidemiology, social, and economic implications. Kidney Int Suppl. 2005;98:S7-s10. https://doi.org/10.1111/j.1523-1755.2005.09801.x.
Gaitonde DY, Cook DL, Rivera IM. Chronic kidney disease: detection and evaluation. Am Fam Physician. 2017;96(12):776–83.
Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Int Med. 2013;158(11):825–30. https://doi.org/10.7326/0003-4819-158-11-201306040-00007.
Akchurin OM. Chronic kidney disease and dietary measures to improve outcomes. Pediatr Clin N Am. 2019;66(1):247–67. https://doi.org/10.1016/j.pcl.2018.09.007.
Vos T, Allen C, Arora M, Barber RM, Bhutta ZA, Brown A, Carter A, Casey DC, Charlson FJ, Chen AZ, Coggeshall M. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet (London, England). 2016;388(10053):1545–602. https://doi.org/10.1016/s0140-6736(16)31678-6.
Chung KW, Dhillon P, Huang S, Sheng X, Shrestha R, Qiu C, et al. Mitochondrial damage and activation of the STING pathway lead to renal inflammation and fibrosis. Cell Metab. 2019;30(4):784-99.e5. https://doi.org/10.1016/j.cmet.2019.08.003.
Liu M, Ning X, Li R, Yang Z, Yang X, Sun S, et al. Signalling pathways involved in hypoxia-induced renal fibrosis. J Cell Mol Med. 2017;21(7):1248–59. https://doi.org/10.1111/jcmm.13060.
Bülow RD, Boor P. Extracellular matrix in kidney fibrosis: more than just a scaffold. J Histochem Cytochem Off J Histochem Soc. 2019;67(9):643–61. https://doi.org/10.1369/0022155419849388.
Sun YB, Qu X, Caruana G, Li J. The origin of renal fibroblasts/myofibroblasts and the signals that trigger fibrosis. Differ Res Biol Divers. 2016;92(3):102–7. https://doi.org/10.1016/j.diff.2016.05.008.
Li R, Guo Y, Zhang Y, Zhang X, Zhu L, Yan T. Salidroside ameliorates renal interstitial fibrosis by inhibiting the TLR4/NF-κB and MAPK Signaling Pathways. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20051103.
Liu Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am Soc Nephrol. 2010;21(2):212–22. https://doi.org/10.1681/asn.2008121226.
Chen YC, Chuang TY, Liu CW, Liu CW, Lee TL, Lai TC, et al. Particulate matters increase epithelial-mesenchymal transition and lung fibrosis through the ETS-1/NF-κB-dependent pathway in lung epithelial cells. Part Fibre Toxicol. 2020;17(1):41. https://doi.org/10.1186/s12989-020-00373-z.
Hickson LJ, Eirin A, Lerman LO. Challenges and opportunities for stem cell therapy in patients with chronic kidney disease. Kidney Int. 2016;89(4):767–78. https://doi.org/10.1016/j.kint.2015.11.023.
Zhuang Q, Ma R, Yin Y, Lan T, Yu M, Ming Y. Mesenchymal stem cells in renal fibrosis: the flame of cytotherapy. Stem Cells Int. 2019. https://doi.org/10.1155/2019/8387350.
Hosseini S, Taghiyar L, Safari F, Baghaban EM. Regenerative medicine applications of mesenchymal stem cells. Adv Exp Med Biol. 2018;1089:115–41. https://doi.org/10.1007/5584_2018_213.
Cagliani J, Grande D, Molmenti EP, Miller EJ, Rilo HLR. Immunomodulation by mesenchymal stromal cells and their clinical applications. J Stem Cell Regen Biol. 2017. https://doi.org/10.15436/2471-0598.17.022.
Marycz KTN, GolonKa P. The therapeutic effect of autogenic adipose derived stem cells combined with autogenic platelet rich plasma in tendons disorders hi horses in vitro and in vivo research. J Anim Vet Adv. 2012;11(23):4324–31.
Marycz KGJ, Wrzeszcz K, Golonka P. Adipose stem cell combined with plasma-based implant bone tissue differentiation in vitro and in a horse with a phalanx digitalis distalis fracture: a case report. Vet Med. 2012;57:610–7.
Semedo P, Correa-Costa M, Antonio Cenedeze M, Maria Avancini Costa Malheiros D, Antonia dos Reis M, Shimizu MH, et al. Mesenchymal stem cells attenuate renal fibrosis through immune modulation and remodeling properties in a rat remnant kidney model. Stem Cells (Dayton Ohio). 2009;27(12):3063–73. https://doi.org/10.1002/stem.214.
Yeo RW, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ, et al. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev. 2013;65(3):336–41. https://doi.org/10.1016/j.addr.2012.07.001.
Liu B, Hu D, Zhou Y, Yu Y, Shen L, Long C, et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against renal interstitial fibrosis through ROS-mediated P38MAPK/ERK signaling pathway. Am J Transl Res. 2020;12(9):4998–5014.
Zhang J, Li S, Li L, Li M, Guo C, Yao J, et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinform. 2015;13(1):17–24. https://doi.org/10.1016/j.gpb.2015.02.001.
Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Pérez Lanzón M, Zini N, et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015;6(1):127. https://doi.org/10.1186/s13287-015-0116-z.
Bu H, He D, He X, Wang K. Exosomes: isolation, analysis, and applications in cancer detection and therapy. Chembiochem Eur J Chem Biol. 2019;20(4):451–61. https://doi.org/10.1002/cbic.201800470.
Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci CMLS. 2018;75(2):193–208. https://doi.org/10.1007/s00018-017-2595-9.
Qu Y, Zhang Q, Cai X, Li F, Ma Z, Xu M, et al. Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J Cell Mol Med. 2017;21(10):2491–502. https://doi.org/10.1111/jcmm.13170.
Wang SY, Hong Q, Zhang CY, Yang YJ, Cai GY, Chen XM. miRNAs in stem cell-derived extracellular vesicles for acute kidney injury treatment: comprehensive review of preclinical studies. Stem Cell Res Ther. 2019;10(1):281. https://doi.org/10.1186/s13287-019-1371-1.
Ahmadi M, Rezaie J. Ageing and mesenchymal stem cells derived exosomes: molecular insight and challenges. Cell Biochem Funct. 2021;39(1):60–6. https://doi.org/10.1002/cbf.3602.
Wang B, Yao K, Huuskes BM, Shen HH, Zhuang J, Godson C, et al. Mesenchymal stem cells deliver exogenous MicroRNA-let7c via exosomes to attenuate renal fibrosis. Mol ther J Am Soc Gene Ther. 2016;24(7):1290–301. https://doi.org/10.1038/mt.2016.90.
Cochrane AL, Kett MM, Samuel CS, Campanale NV, Anderson WP, Hume DA, et al. Renal structural and functional repair in a mouse model of reversal of ureteral obstruction. J Am Soc Nephrol. 2005;16(12):3623–30. https://doi.org/10.1681/asn.2004090771.
Zhou J, Lin Y, Kang X, Liu Z, Zhang W, Xu F. microRNA-186 in extracellular vesicles from bone marrow mesenchymal stem cells alleviates idiopathic pulmonary fibrosis via interaction with SOX4 and DKK1. Stem Cell Res Ther. 2021;12(1):96. https://doi.org/10.1186/s13287-020-02083-x.
Lan HY. Diverse roles of TGF-β/Smads in renal fibrosis and inflammation. Int J Biol Sci. 2011;7(7):1056–67. https://doi.org/10.7150/ijbs.7.1056.
Ji C, Zhang J, Zhu Y, Shi H, Yin S, Sun F, et al. Exosomes derived from hucMSC attenuate renal fibrosis through CK1δ/β-TRCP-mediated YAP degradation. Cell Death Dis. 2020;11(5):327. https://doi.org/10.1038/s41419-020-2510-4.
Liao J, Liu R, Shi YJ, Yin LH, Pu YP. Exosome-shuttling microRNA-21 promotes cell migration and invasion-targeting PDCD4 in esophageal cancer. Int J Oncol. 2016;48(6):2567–79. https://doi.org/10.3892/ijo.2016.3453.
Thongboonkerd V. Roles for exosome in various kidney diseases and disorders. Front Pharmacol. 2019;10:1655. https://doi.org/10.3389/fphar.2019.01655.
Yi YW, Lee JH, Kim SY, Pack CG, Ha DH, Park SR, et al. Advances in analysis of biodistribution of exosomes by molecular imaging. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21020665.
Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells. Int J Mol Sci. 2014;15(3):4142–57. https://doi.org/10.3390/ijms15034142.
He X, Dong Z, Cao Y, Wang H, Liu S, Liao L, et al. MSC-derived exosome promotes M2 polarization and enhances cutaneous wound healing. Stem Cells Int. 2019. https://doi.org/10.1155/2019/7132708.
Matsui F, Babitz SK, Rhee A, Hile KL, Zhang H, Meldrum KK. Mesenchymal stem cells protect against obstruction-induced renal fibrosis by decreasing STAT3 activation and STAT3-dependent MMP-9 production. Am J Physiol Ren Physiol. 2017;312(1):F25-f32. https://doi.org/10.1152/ajprenal.00311.2016.
Eirin A, Zhu XY, Puranik AS, Tang H, McGurren KA, van Wijnen AJ, et al. Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int. 2017;92(1):114–24. https://doi.org/10.1016/j.kint.2016.12.023.
Yao K, Ricardo SD. Mesenchymal stem cells as novel micro-ribonucleic acid delivery vehicles in kidney disease. Nephrology (Carlton). 2016;21(5):363–71. https://doi.org/10.1111/nep.12643.
Patel V, Noureddine L. MicroRNAs and fibrosis. Curr Opin Nephrol Hypertens. 2012;21(4):410–6. https://doi.org/10.1097/MNH.0b013e328354e559.
Wang B, Zhang C, Zhang A, Cai H, Price SR, Wang XH. MicroRNA-23a and MicroRNA-27a mimic exercise by ameliorating CKD-induced muscle atrophy. J Am Soc Nephrol. 2017;28(9):2631–40. https://doi.org/10.1681/asn.2016111213.
Wang Y, Guo YF, Fu GP, Guan C, Zhang X, Yang DG, et al. Protective effect of miRNA-containing extracellular vesicles derived from mesenchymal stromal cells of old rats on renal function in chronic kidney disease. Stem Cell Res Ther. 2020;11(1):274. https://doi.org/10.1186/s13287-020-01792-7.
Lei GS, Kline HL, Lee CH, Wilkes DS, Zhang C. Regulation of collagen V expression and epithelial-mesenchymal transition by miR-185 and miR-186 during idiopathic pulmonary fibrosis. Am J Pathol. 2016;186(9):2310–6. https://doi.org/10.1016/j.ajpath.2016.04.015.
Gooch JL, King C, Francis CE, Garcia PS, Bai Y. Cyclosporine A alters expression of renal microRNAs: New insights into calcineurin inhibitor nephrotoxicity. PLoS ONE. 2017;12(4): e0175242. https://doi.org/10.1371/journal.pone.0175242.
Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659):498–503. https://doi.org/10.1038/nature22341.
Huuskes BM, Wise AF, Cox AJ, Lim EX, Payne NL, Kelly DJ, et al. Combination therapy of mesenchymal stem cells and serelaxin effectively attenuates renal fibrosis in obstructive nephropathy. FASEB J Off Publ Fed Am Soc Exp Biol. 2015;29(2):540–53. https://doi.org/10.1096/fj.14-254789.
Ma TT, Meng XM. TGF-β/Smad and renal fibrosis. Adv Exp Med Biol. 2019;1165:347–64. https://doi.org/10.1007/978-981-13-8871-2_16.
Pons M, Koniaris LG, Moe SM, Gutierrez JC, Esquela-Kerscher A, Zimmers TA. GDF11 induces kidney fibrosis, renal cell epithelial-to-mesenchymal transition, and kidney dysfunction and failure. Surgery. 2018;164(2):262–73. https://doi.org/10.1016/j.surg.2018.03.008.
Schiffer M, von Gersdorff G, Bitzer M, Susztak K, Böttinger EP. Smad proteins and transforming growth factor-beta signaling. Kidney Int Suppl. 2000;77:S45-52. https://doi.org/10.1046/j.1523-1755.2000.07708.x.
Yu Y, Hu D, Zhou Y, Xiang H, Liu B, Shen L, et al. Human umbilical cord mesenchymal stem cell attenuates renal fibrosis via TGF-β/Smad signaling pathways in vivo and in vitro. Eur J Pharmacol. 2020;883: 173343. https://doi.org/10.1016/j.ejphar.2020.173343.
Wang H, Wang B, Zhang A, Hassounah F, Seow Y, Wood M, et al. Exosome-mediated miR-29 transfer reduces muscle atrophy and kidney fibrosis in mice. Mol Ther J Am Soc Gene Ther. 2019;27(3):571–83. https://doi.org/10.1016/j.ymthe.2019.01.008.
Funding
National Natural Scientific Foundation of China (No. 81370868) and Key R & D Program of Jiangsu Province, China (No BE2019712).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Ethical approval
All experiments were approved by the Institutional Ethical Committee of the Affiliated Zhongda Hospital, Southeast University, which adheres to the National Institutes of Health Guide for the Care and Use of laboratory animals.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

13577_2021_617_MOESM1_ESM.jpg
Supplementary file1 Supplementary Figure 1 MiR-186-5p level in exosomes derived from transfected MSCs. (A) MiR-186-5p level in MSCs transfected with miR-186-5p antagomir or NC was analyzed by RT-qPCR. **P < 0.01 compared with the NC group. (B) MSCs were transfected with NC or miR-186-5p antagomir for 48 h. RT-qPCR analysis of miR-186-5p levels in exosomes isolated from the CM of transfected MSCs. **P < 0.01 compared with the MSCs/NC group. (C) The level of miR-186-5p in NRK52E cells co-cultured with MSCs transfected with NC or miR-186-5p antagomir. **P < 0.01 compared with the NRK52E + MSCs/NC group. MSCs/NC, MSCs transfected with NC; MSCs/anti-miR-186-5p, MSCs transfected with miR-186-5p antagomir; NC, negative control (JPG 2763 kb)

13577_2021_617_MOESM2_ESM.jpg
Supplementary file2 Supplementary Figure 2 The uptake of exosomes. (A, B) NRK52E cells were co-cultured with exosomes derived from MSCs/miR-186-5p or from MSCs/NC for 48 h. The PKH26 fluorescence signal in cells was observed by confocal microscopy. MSCs/NC-Exo, exosomes derived from MSCs transfected with NC; MSCs/miR-186-5p-Exo, exosomes derived from MSCs transfected with miR-186-5p agomir (JPG 4563 kb)

13577_2021_617_MOESM3_ESM.jpg
Supplementary file3 Supplementary Figure 3 MSCs-secreted anti-miR-186-5p promoted the EMT and apoptosis in TGF-β1-treated NRK52E cells. NRK52E cells were treated with exosomes isolated from the CM of MSCs transfected with NC or miR-186-5p antagomir for 72 h in the presence of TGF-β1. (A) Expressions of collagen III, fibronectin and α-SMA in NRK52E cells were detected with western blotting. (B) Apoptotic cells were detected with Annexin V and PI double staining. (C) Expressions of Bax and active caspase 3 in NRK52E cells were detected with western blotting. *P < 0.05, **P < 0.01. MSCs/NC-Exo, exosomes derived from MSCs transfected with NC; MSCs/anti-miR-186-5p-Exo, exosomes derived from MSCs transfected with miR-186-5p antagomir (JPG 4586 kb)

13577_2021_617_MOESM4_ESM.jpg
Supplementary file4 Supplementary Figure 4 MiR-186-5p agomir alleviated renal fibrosis in UUO mice. (A, B) The levels of BUN and CR in serum of mice were detected by ELISA. (C) H&E staining assay was used to determine kidney injury in UUO kidneys. (D) Masson’s trichrome staining assay was used to determine collagen deposition and renal fibrosis in UUO kidneys. **P < 0.01 compared with sham group; #P < 0.05, ##P < 0.01 compared with NC + UUO group (JPG 9340 kb)
Rights and permissions
About this article
Cite this article
Yang, Y., Wang, J., Zhang, Y. et al. Exosomes derived from mesenchymal stem cells ameliorate renal fibrosis via delivery of miR-186-5p. Human Cell 35, 83–97 (2022). https://doi.org/10.1007/s13577-021-00617-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-021-00617-w