Abstract
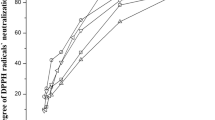
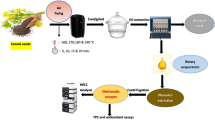
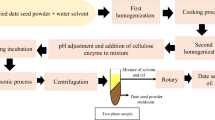
The baru almond stands out as almond rich in proteins, lipids, fibers, bioactive compounds, and antioxidant potential. This study aimed to optimize the extraction of fixed oil from baru seed (Dipteryx alata), performed by the hydraulic press and Soxhlet using eco-friendly solvents (ethanol, 2-methyl tetrahydrofuran (2-METHF), and d-limonene), using preliminary treatment with ultrasound and microwaves, as well as carrying out the chemical and functional oil and evaluate its stability during 60 days of storage at 25 °C. Evaluating the combinations of extraction methods and solvents, ethanol was the solvent that presented the best results. The ultrasound method as a pretreatment in 30 min, followed by hydraulic pressing, was the one that presented the best results in oil yield and quality. Therefore, this method was adopted for the other stages of this study. Regarding the chemical composition of the oil, it was rich in unsaturated fatty acids, the main fatty acid being oleic acid at 45.90%, followed by linoleic acid at 25.96%. Regarding oxidative stability, the oil remained stable during 30 days of storage at 25 °C. During the days of storage, there were changes in the peroxide index (of 0.15 for 4.32 meq Kg−1), in the composition of polyunsaturated fatty acids (of 26.04 for 25.03%), and a reduction in the total tocopherols (of 10.94 for 7.43 mg 100 g−1) present in the oil. Finally, ultrasound has shown promise in extracting baru oil. Still, special methods are needed to protect the extracted oils from pro-oxidative factors.
Graphical abstract

Similar content being viewed by others
Data availability
The article material presents all data relevant to this study.
References
Herculano LS, Lukasievicz GVB, Sehn E, Torquato AS, Belançon MP, Savi E, Kimura NM, Malacarne LC, Baesso ML, Astrath NGC (2021) The correlation of physicochemical properties of edible vegetable oils by chemometric analysis of spectroscopic data. Spectrochim Acta A: Mol Biomol Spectrosc 245:118877. https://doi.org/10.1016/j.saa.2020.118877
Morais RA, Teixeira GL, Ferreira SRS, Cifuentes A, Block JM (2022) Nutritional composition and bioactive compounds of native Brazilian fruits of the Arecaceae family and its potential applications for health promotion. Nutrients 14:4009. https://doi.org/10.3390/nu14194009
El Khetabi A, Lahlali R, Ezrari S, Radouane N, Nadia L, Banani H, Barka EA (2022) Role of plant extracts and essential oils in fighting against postharvest fruit pathogens and extending fruit shelf life: a review. Trends Food Sci Technol 120:402–417. https://doi.org/10.1016/j.tifs.2022.01.009
Olagunju AI, Adelakun OS, Olawoyin MS (2022) The effect of rice bran extract on the quality indices, physicochemical properties and oxidative stability of soybean oil blended with various oils. Measurement: Food 6:100032. https://doi.org/10.1016/j.meafoo.2022.100032
Perumal AB, Huang L, Nambiar RB, He Y, Li X, Sellamuthu PS (2022) Application of essential oils in packaging films for the preservation of fruits and vegetables: a review. Food Chem 375:131810. https://doi.org/10.1016/j.foodchem.2021.131810
Oliveira ÉR, Silva RF, Santos PR, Queiroz F (2019) Potential of alternative solvents to extract biologically active compounds from green coffee beans and its residue from the oil industry. Food Bioprod Process 115:47–58. https://doi.org/10.1016/j.fbp.2019.02.005
Santos KA, Silva EA, Silva C (2021) Ultrasound-assisted extraction of favela (Cnidoscolus quercifolius) seed oil using ethanol as a solvent. J Food Process Preserv 45:e15497. https://doi.org/10.1111/jfpp.15497
Potrich E, Miyoshi SC, Machado PF, Furlan FF, Ribeiro MP, Tardioli PW, Giordano RC (2020) Replacing hexane by ethanol for soybean oil extraction: modeling, simulation, and techno-economic-environmental analysis. J Clean Prod 244:118660. https://doi.org/10.1016/j.jclepro.2019.118660
Amarante RC, Oliveira PM, Schwantes FK, Morón-Villarreyes JA (2014) Oil extraction from castor cake using ethanol: kinetics and thermodynamics. Ind Eng Chem Res 53:6824–6829. https://doi.org/10.1021/ie500508n
Baümler ER, Carrín ME, Carelli AA (2016) Extraction of sunflower oil using ethanol as solvent. J Food Eng 178:190–197. https://doi.org/10.1016/j.jfoodeng.2016.01.020
Comerlatto A, Voll FA, Daga AL, Fontana É (2021) Mass transfer in soybean oil extraction using ethanol/isopropyl alcohol mixtures. Int J Heat Mass Transfer 165:120630. https://doi.org/10.1016/j.ijheatmasstransfer.2020.120630
Stevanato N, Silva C (2019) Radish seed oil: ultrasound-assisted extraction using ethanol as solvent and assessment of its potential for ester production. Ind Crops Prod 132:283–291. https://doi.org/10.1016/j.indcrop.2019.02.032
Ferreira MC, Gonçalves D, Bessa LC, Rodrigues CE, Meirelles AJ, Batista EA (2022) Soybean oil extraction with ethanol from multiple-batch assays to reproduce a continuous, countercurrent, and multistage equipment. Chem Eng Process-Process Intensific 170:108659. https://doi.org/10.1016/j.cep.2021.108659
Tarazanov SV, Lukyanova VA, Ilin DY, Dorofeeva OV, Druzhinina AI, Pimenova SM (2022) Enthalpy of formation of 2-methyltetrahydrofuran: experimental and computational study. J Chem Thermodyn 165:106651. https://doi.org/10.1016/j.jct.2021.106651
Sicaire AG, Vian MA, Fine F, Carré P, Tostain S, Chemat F (2016) Ultrasound induced green solvent extraction of oil from oleaginous seeds. Ultrasond Sonochem 31:319–329. https://doi.org/10.1016/j.ultsonch.2016.01.011
Rodrigues SN, Mishra S, Sahu JK, Naik SN (2022) Effect of ultrasound-assisted extraction on efficiency, antioxidant activity, and physicochemical properties of sea buckthorn (Hippophae salicipholia) seed oil. LWT - Food Sci Technol 153:112386. https://doi.org/10.1016/j.lwt.2021.112386
Souza CLM, Lemos MCM, Sanches EA, Silva LS, Bezerra JA, Aguiar JPL, Campelo PH (2020) Improvement of the bioaccessibility of bioactive compounds from Amazon fruits treated using high energy ultrasound. Ultrason Sonochem 67:105148. https://doi.org/10.1016/j.ultsonch.2020.105148
Liu HM, Yao YG, Ma YX, Wang XD (2020) Ultrasound-assisted desolventizing of fragrant oil from red pepper seed by subcritical propane extraction. Ultrason Sonochem 63:104943. https://doi.org/10.1016/j.ultsonch.2019.104943
Moraes C, Anjos JL, Maruno M, Alonso A, Filho PR (2018) Development of lamellar gel phase emulsion containing baru oil (Dipteryx alata Vog.) as a prospective delivery system for cutaneous application. Asian J Pharm Sci 13:183–190. https://doi.org/10.1016/j.ajps.2017.09.003
Silva JS, Ferreira NBS, Asquieri ER, Damiani C, Asquieri EMDAR (2020) Chemical monitoring of baru (Dipteryx alata Vog.) pulp fermented beverage. Food Sci Technol 41:155–162. https://doi.org/10.1590/fst.14420
Pineli LLO, Carvalho MV, Aguiar LA, Oliveira GT, Celestino SMC, Assunção RBB, Chiarello MD (2015) Use of baru (Brazilian almond) waste from physical extraction of oil to produce flour and cookies. LWT Food Sci Technol 60:50–55. https://doi.org/10.1016/j.lwt.2014.09.035
Hartman L, Lago RC (1973) Rapid preparation of fatty acid methyl esters from lipids. Lab Pract 22:475–476
AOCS (2009) Official methods and recommended practices of the American Oil Chemist’ Society, 6th. AOCS, Champaign
Ulbricht TLV, Southgate DAT (1991) Coronary heart disease: seven dietary factors. Lancet 338:985–992. https://doi.org/10.1016/0140-6736(91)91846-M
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture 16: 144–158. Available: https://www.ajevonline.org/content/16/3/144.short. Access: 14 march. 2023
Rodriguez-Amaya DB (2001) A guide to carotenoid analysis in foods; ILSI Press: Washington, DC, USA. Available: https://pdf.usaid.gov/pdf_docs/pnacq929.pdf. Access: 11 January. 2023
Zhu Y, Wilkinson KL, Wirthensohn MG (2015) Lipophilic antioxidant content of almonds (Prunus dulcis): a regional and varietal study. J Food Compos Anal 39:120–127. https://doi.org/10.1016/j.jfca.2014.12.003
Kozłowska M, Gruczyńska E, Ścibisz I, Rudzińska M (2016) Fatty acids and sterols composition, and antioxidant activity of oils extracted from plant seeds. Food Chem 213:450–456. https://doi.org/10.1016/j.foodchem.2016.06.102
Siddiqui SA, Pahmeyer MJ, Assadpour E, Jafari SM (2022) Extraction and purification of d-limonene from orange peel wastes: recent advances. Ind Crops Prod 177:114484. https://doi.org/10.1016/j.indcrop.2021.114484
Codex Alimentarius (2010) Fats, oils and related products. Food Agricult. Organization, Rome
Vicente J, Carvalho MG, Rojas EEG (2015) Fatty acids profile of Sacha Inchi oil and blends by 1H NMR and GC-FID. Food Chem 181:215–221. https://doi.org/10.1016/j.foodchem.2015.02.092
Hu B, Li Y, Song J, Li H, Zhou Q, Li C, Zhang Z, Liu Y, Liu A, Zhang Q, Liu S, Luo Q (2020) Oil extraction from tiger nut (Cyperus esculentus L.) using the combination of microwave-ultrasonic assisted aqueous enzymatic method - design, optimization and quality evaluation. J Chromatogr A 1:1627. https://doi.org/10.1016/j.chroma.2020.461380
Jesus SS, Filho RM (2020) Recent advances in lipid extraction using green solvents. Renew Sustain Energy Rev 133:110289. https://doi.org/10.1016/j.rser.2020.110289
Panadare DC, Rathod VK (2020) Process intensification of three phase partition for extraction of custard apple seed oil using microwave pretreatment. Chem Eng Process-Process Intensif 157:108095. https://doi.org/10.1016/j.cep.2020.108095
Rigane G, Yahyaoui A, Acar A, Mnif S, Salem RB, Arslan D (2020) Change in some quality parameters and oxidative stability of olive oils with regard to ultrasound pretreatment, depitting and water addition. Biotechnol Reports 26:4–9. https://doi.org/10.1016/j.btre.2020.e00442
Bakhshabadi H, Mirzaei H, Ghodsvali A, Jafari SM, Ziaiifar AM, Farzaneh V (2017) The effect of microwave pretreatment on some physico-chemical properties and bioactivity of Black cumin seeds’ oil. Ind Crops Prod 97:1–9. https://doi.org/10.1016/j.indcrop.2016.12.005
Polmann G, Teixeira GL, Santos PH, Rivera GÁ, Ibañez E, Cifuentes A, Block JM (2023) Chemical characterization of gurguéia nut (Dipteryx lacunifera Ducke) and press cake oil obtained by hydraulic pressing and supercritical extraction. Biomass Convers Biorefin 1:1–16. https://doi.org/10.1007/s13399-023-04042-x
Paucar LOC, Tobón JFO, Johner JCF, Meireles MAA (2021) A comparative and economic study of the extraction of oil from Baru (Dipteryx alata) seeds by supercritical CO2 with and without mechanical pressing. Heliyon 7:e05971. https://doi.org/10.1016/j.heliyon.2021.e05971
Ferreira MJA, Mota MFS, Mariano RGB, Freitas SP (2021) Evaluation of liquid-liquid extraction to reducing the acidity index of the tucuma (Astrocaryum vulgare Mart.) pulp oil. Sep Purif Technol 257:117894. https://doi.org/10.1016/j.seppur.2020.117894
Fetzer DL, Cruz PN, Hamerski F, Corazza ML (2018) Extraction of baru (Dipteryx alata vogel) seed oil using compressed solvents technology. J Supercrit Fluids 137:23–33. https://doi.org/10.1016/j.supflu.2018.03.004
Reis MÁ, Novaes RD, Baggio SR, Luiz A, Viana M, Cesar B, Salles C, Maris S, Rodrigues MR, Borges F, Paula DA (2018) Hepatoprotective and antioxidant activities of oil from Baru almonds (Dipteryx alata Vog.) in a preclinical model of lipotoxicity and dyslipidemia. Evid-Based Complement Alternat Med 11:8376081. https://doi.org/10.1155/2018/8376081
Serra JL, Rodrigues AMC, Freitas RA, Meirelles AJA, Darnet SH, Silva LHM (2019) Alternative sources of oils and fats from Amazonian plants: fatty acids, methyl tocols, total carotenoids and chemical composition. Food Res Int 116:12–19. https://doi.org/10.1016/j.foodres.2018.12.028
Kogawa NRDA, Arruda EJ, Micheletti AC, Matos MDFC, Oliveira LCS, Lima DP, Carvalho NCP, Oliveira PD, Cunha MDC, Ojeda M, Beatriz A (2015) Synthesis, characterization, thermal behavior, and biological activity of ozonides from vegetable oils. RSC Adv 5:65427–65436. https://doi.org/10.1039/c5ra02798e
Brasil RV, Cavallieri ÂLF, Costa ALM, Gonçalves MÁB (2011) Caracterização física e química do óleo de pequi exposto a diferentes condições de armazenamento. Jornal Da Ciência - SBPC 14. Available in <http://www.sbpcnet.org.br/livro/63ra/conpeex/pibic/trabalhos/renata_v.pdf>
Ponte FAF, Rodrigues JS, Malveira JQ, Filho JASR, Albuquerque MCG (2017) Avaliação físico-química dos óleos de babaçu (Orbignya speciosa) e coco (Cocos nucifera) com elevado índice de acidez e dos ácidos graxos (C6 a C16). Scientia Plenan13: 1–8. https://doi.org/10.14808/sci.plena.2017.085301
Figueiredo PS, Martins TN, Ravaglia LM, Alcantara GB, Guimarães RDCA, Freitas KDC, Hiane PA (2022) Linseed, baru, and coconut oils: NMR-based metabolomics, leukocyte infiltration potential in vivo, and their oil characterization. Are there still controversies? Nutrients 14:1161. https://doi.org/10.3390/nu14061161
Marques FG, Neto JRO, Cunha LC, Paula JR, Bara MTF (2015) Identification of terpenes and phytosterols in Dipteryx alata (baru) oil seeds obtained through pressing. Rev Bras 25:522–525. https://doi.org/10.1016/j.bjp.2015.07.019
Bezerra CV, Manoel A, Oliveira PD, Albuquerque D, Helena L (2017) Technological properties of amazonian oils and fats and their applications in the food industry. Food Chem 221:1466–1473. https://doi.org/10.1016/j.foodchem.2016.11.004
Pineli L, Oliveira G, Mendonça M, Borgo L, Freire É, Celestino S, Botelho R (2015) Tracing chemical and sensory characteristics of baru oil during storage under nitrogen. LWT-Food Sci Technol 62:976–982. https://doi.org/10.1016/j.lwt.2015.02.015
Polette CMV, Ramos PR, Gonçalves CB, Oliveira AL (2021) Determination of free fatty acids in crude vegetable oil samples obtained by high-pressure processes. Food Chem X 12:100166. https://doi.org/10.1016/j.fochx.2021.100166
Naebi M, Torbati M, Damirchi SA, Siabi S, Savage GP (2022) Changes in physicochemical properties of cold press extracted oil from Balangu (Lallemantia peltata) seeds during storage. J Food Compos Anal 107:104358. https://doi.org/10.1016/j.jfca.2021.104358
Alarcon RT, Gaglieri C, Lamb KJ, North M, Bannach G (2020) Spectroscopic characterization and thermal behavior of baru nut and macaw palm vegetable oils and their epoxidized derivatives. Ind Crops Prod 154:112585. https://doi.org/10.1016/j.indcrop.2020.112585
Gupta M (2017) Practical guide to vegetable oil processing, 2nd edn. Academic Press and AOCS Press, Lynnwood. https://doi.org/10.1016/B978-1-63067-050-4.00018-0
Ishak I, Hussain N, Coorey R, Ghani MA (2021) Optimization and characterization of chia seed (Salvia hispanica L.) oil extraction using supercritical carbon dioxide. J CO2 Utiliz 45:101430. https://doi.org/10.1016/j.jcou.2020.101430
Santos CS, Cunha SC, Casal S (2017) Deep or air frying? A comparative study with different vegetable oils. Eur J Lipid Sci Technol 119:1600375. https://doi.org/10.1002/ejlt.201600375
Musakhanian J, Rodier JD, Dave M (2022) Oxidative stability in lipid formulations: a review of the mechanisms, drivers, and inhibitors of oxidation. AAPS PharmSciTech 23:151. https://doi.org/10.1208/s12249-022-02282-0
Funding
This work was partially funded by the Coordination for the Improvement of Higher Education Personnel—Brazil (CAPES)—Financial Code 001. G.A.S. Martins received funding with the CAPES/Brazil no.: 88881.200497/2018–01, PROCAD-AM 1707/2018.
Author information
Authors and Affiliations
Contributions
Greice Folis Dagostin Santinoni: conceptualization, methodology, validation, formal analysis, original draft, investigation; Rômulo Alves Morais: methodology, validation, original draft, investigation, review and editing; Gabriela Fonsêca Leal: methodology, validation, formal analysis; Vinícius Soares dos Reis: methodology, validation, formal analysis; Glêndara Aparecida de Souza Martins: formal analysis, conceptualization, resources, supervision; Clarissa Damiani: conceptualization, resources, supervision, project administration, review and editing.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Santinoni, G.F.D., Morais, R.A., Leal, G.F. et al. Agroindustrial valorization of baru almond oil (Dipteryx alata) through sustainable techniques: a study on nutritional quality, oxidative stability, fatty acid, and tocopherol profile. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04578-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04578-y