Abstract
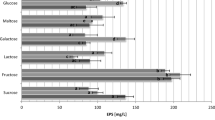
As one of the most promising groups of microbes, lactic acid bacteria (LAB) can synthesize metabolites that can be used in different industries over the world, mainly in the pharmaceutical, food, and dairy industries. In this study, a novel exopolysaccharide was extracted and isolated from the probiotic strain Lactobacillus reuteri B2, and assessed on biological activity and its possible application as a biosorbent for the removal of Ni2+ ions from contaminated water. New exopolysaccharide was characterized using FTIR, SEM, XRD, NMR, MALDI-TOF MS, and TGA/DTG analysis. Biological assays included antioxidative activity, cytotoxic assay, and adhesion assay of L. reuteri B2 to HT29 cells. Our hypothesis was that if this exopolysaccharide is nontoxic, it can be used as a novel biomaterial for the possible application of the removal of Ni2+ ions from contaminated water. The scavenging effect of nontoxic exopolysaccharide was 76% at 2 mg/mL using ABTS assay, in biological assays, while the removal efficiency of nickel from the aqueous solution was 92.96% in biosorption study. According to these results, this exopolysaccharide can be considered a very promising biomaterial for potential application in different industries, from pharmacy to wastewater treatments.
Graphical abstract











Similar content being viewed by others
Data availability
Additional information can be found in the Supplementary Material.
References
Hooshdar P, Kermanshahi RK, Ghadam P, Khosravi-Darani K (2020) A review on production of exopolysaccharide and biofilm in probiotics like lactobacilli and methods of analysis. Biointerface Res Appl Chem 10:6058–6075. https://doi.org/10.33263/BRIAC105.60586075
Mehta K, Shukla A, Saraf M (2021) Articulating the exuberant intricacies of bacterial exopolysaccharides to purge environmental pollutants. Heliyon 7:e08446. https://doi.org/10.1016/j.heliyon.2021.e08446
Segers ME, Lebeer S (2014) Towards a better understanding of Lactobacillus rhamnosus GG - host interactions. Microb Cell Fact 13:1–16. https://doi.org/10.1186/1475-2859-13-S1-S7
Freitas F, Alves VD, Reis MAM (2011) Advances in bacterial exopolysaccharides: from production to biotechnological applications. Trends Biotechnol 29:388–398. https://doi.org/10.1016/j.tibtech.2011.03.008
Abid Y, Casillo A, Gharsallah H et al (2018) Production and structural characterization of exopolysaccharides from newly isolated probiotic lactic acid bacteria. Int J Biol Macromol 108:719–728. https://doi.org/10.1016/j.ijbiomac.2017.10.155
Chen YC, Wu YJ, Hu CY (2019) Monosaccharide composition influence and immunomodulatory effects of probiotic exopolysaccharides. Int J Biol Macromol 133:575–582. https://doi.org/10.1016/j.ijbiomac.2019.04.109
Ashok Kumar Nadda Sajna K. V. Swati Sharma (2021) Exopolysaccharides as novel and significant biomaterials, Springer Series on Polymer and Composite Materials (SSPCM), ISBN: 978–3–030–75289–7
Mohite BV, Koli SH, Narkhede CP et al (2017) Prospective of microbial exopolysaccharide for heavy metal exclusion. Appl Biochem Biotechnol 183:582–600. https://doi.org/10.1007/s12010-017-2591-4
Ibrahim IM, Konnova SA, Sigida EN et al (2020) Bioremediation potential of a halophilic Halobacillus sp. strain, EG1HP4QL: exopolysaccharide production, crude oil degradation, and heavy metal tolerance. Extremophiles 24:157–166. https://doi.org/10.1007/s00792-019-01143-2
Fredrik Levander, Malin Svensson and PR (2002) Enhanced exopolysaccharide production by metabolic engineering of Streptococcus thermophilus. Appl Environ Microbiol. https://doi.org/10.1128/AEM.68.11.5656-5662.2002
Concórdio-Reis P, Reis MAM, Freitas F (2020) Biosorption of heavy metals by the bacterial exopolysaccharide fucopol. Appl Sci 10. https://doi.org/10.3390/APP10196708
Zeraatkar AK, Ahmadzadeh H, Talebi AF et al (2016) Potential use of algae for heavy metal bioremediation, a critical review. J Environ Manage 181:817–831. https://doi.org/10.1016/j.jenvman.2016.06.059
Lu H, Shi X, Costa M, Huang C (2005) Carcinogenic effect of nickel compounds. Mol Cell Biochem 279:45–67. https://doi.org/10.1007/s11010-005-8215-2
Shrestha R, Ban S, Devkota S et al (2021) Technological trends in heavy metals removal from industrial wastewater: a review. J Environ Chem Eng. 9:105688. https://doi.org/10.1016/j.jece.2021.105688
Dobrowolski R, Szcześ A, Czemierska M, Jarosz-Wikołazka A (2017) Studies of cadmium(II), lead(II), nickel(II), cobalt(II) and chromium(VI) sorption on extracellular polymeric substances produced by Rhodococcus opacus and Rhodococcus rhodochrous. Bioresour Technol 225:113–120. https://doi.org/10.1016/j.biortech.2016.11.040
Li H, Dong W, Liu Y et al (2019) Enhanced biosorption of nickel ions on immobilized surface-engineered yeast using nickel-binding peptides. Front Microbiol 10. https://doi.org/10.3389/fmicb.2019.01254
Popović M, Stojanović M, Veličković Z et al (2021) Characterization of potential probiotic strain, L. reuteri B2, and its microencapsulation using alginate-based biopolymers. Int J Biol Macromol 183:423–434. https://doi.org/10.1016/j.ijbiomac.2021.04.177
Bajpai VK, Majumder R, Rather IA, Kim K (2016) Extraction, isolation and purification of exopolysaccharide from lactic acid bacteria using ethanol precipitation method. Bangladesh J Pharmacol 11:573–576. https://doi.org/10.3329/bjp.v11i3.27170
Sims IM, Frese SA, Walter J et al (2011) Structure and functions of exopolysaccharide produced by gut commensal Lactobacillus reuteri. 100–23. ISME J 5:1115–1124. https://doi.org/10.1038/ismej.2010.201
Xu Y, Cui Y, Wang X et al (2019) Purification, characterization and bioactivity of exopolysaccharides produced by Lactobacillus plantarum KX041. Int J Biol Macromol 128:480–492. https://doi.org/10.1016/j.ijbiomac.2019.01.117
Tiwari ON, Mondal A, Bhunia B et al (2019) Purification, characterization and biotechnological potential of new exopolysaccharide polymers produced by cyanobacterium Anabaena sp. CCC 745. Polymer (Guildf). 178:121695. https://doi.org/10.1016/j.polymer.2019.121695
Gonzalez-Gil G, Thomas L, Emwas AH et al (2015) NMR and MALDI-TOF MS based characterization of exopolysaccharides in anaerobic microbial aggregates from full-scale reactors. Sci Rep 5:1–12. https://doi.org/10.1038/srep14316
Mitra I, Mukherjee S, Reddy Venkata PB et al (2016) Benzimidazole based Pt(II) complexes with better normal cell viability than cisplatin: synthesis, substitution behavior, cytotoxicity, DNA binding and DFT study. RSC Adv 6:76600–76613. https://doi.org/10.1039/c6ra17788c
Prior RL, Wu X, Schaich K (2005) Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem 53:4290–4302. https://doi.org/10.1021/jf0502698
Milošević MD, Marinković AD, Petrović P et al (2020) Synthesis, characterization and SAR studies of bis(imino)pyridines as antioxidants, acetylcholinesterase inhibitors and antimicrobial agents. Bioorg Chem 102:104073. https://doi.org/10.1016/j.bioorg.2020.104073
Mustafa S, Dilara B, Nargis K et al (2002) Surface properties of the mixed oxides of iron and silica. Colloids Surfaces A Physicochem Eng Asps 205:273–282. https://doi.org/10.1016/S0927-7757(02)00025-0
Li H, Liu L, Cui J et al (2020) High-efficiency adsorption and regeneration of methylene blue and aniline onto activated carbon from waste edible fungus residue and its possible mechanism. RSC Adv 10:14262–14273. https://doi.org/10.1039/d0ra01245a
Wang X, Shao C, Liu L et al (2017) Optimization, partial characterization and antioxidant activity of an exopolysaccharide from Lactobacillus plantarum KX041. Int J Biol Macromol 103:1173–1184. https://doi.org/10.1016/j.ijbiomac.2017.05.118
Charoenwongpaiboon T, Wangpaiboon K, Pichyangkura R et al (2021) Characterization of a nanoparticulate exopolysaccharide from Leuconostoc holzapfelii KM01 and its potential application in drug encapsulation. Int J Biol Macromol 187:690–698. https://doi.org/10.1016/j.ijbiomac.2021.07.174
Wangpaiboon K, Waiyaseesang N, Panpetch P et al (2020) Characterisation of insoluble α-1,3-/α-1,6 mixed linkage glucan produced in addition to soluble α-1,6-linked dextran by glucansucrase (DEX-N) from Leuconostoc citreum ABK-1. Int J Biol Macromol 152:473–482. https://doi.org/10.1016/j.ijbiomac.2020.02.247
Wang J, Ai H, Liu M (2014) Enhanced welan gum production using cane molasses as substrate by Alcaligenes sp ATCC31555. N Biotechnol 31:S40. https://doi.org/10.1016/j.nbt.2014.05.1704
Melo MRS, Feitosa JPA, Freitas ALP, De Paula RCM (2002) Isolation and characterization of soluble sulfated polysaccharide from the red seaweed Gracilaria cornea. Carbohydr Polym 49:491–498. https://doi.org/10.1016/S0144-8617(02)00006-1
Wang J, Zhao X, Yang Y et al (2015) Characterization and bioactivities of an exopolysaccharide produced by Lactobacillus plantarum YW32. Int J Biol Macromol 74:119–126. https://doi.org/10.1016/j.ijbiomac.2014.12.006
Ermiş E, Poyraz E, Dertli E, Yılmaz MT (2020) Optimization of exopolysaccharide production of Lactobacillus brevis E25 using RSM and characterization. Sak Univ J Sci. 151–160. https://doi.org/10.16984/saufenbilder.545929
Wu J, Yan D, Liu Y, et al (2021) Purification, structural characteristics, and biological activities of exopolysaccharide isolated from Leuconostoc mesenteroides SN-8. Front Microbiol 12. https://doi.org/10.3389/fmicb.2021.644226
Meng M, Cheng D, Han L et al (2017) Isolation, purification, structural analysis and immunostimulatory activity of water-soluble polysaccharides from Grifola frondosa fruiting body. Carbohydr Polym 157:1134–1143. https://doi.org/10.1016/j.carbpol.2016.10.082
Chi Z, Su CD, Lu WD (2007) A new exopolysaccharide produced by marine Cyanothece sp. 113. Bioresour Technol 98:1329–1332. https://doi.org/10.1016/j.biortech.2006.05.001
Shuhong Y, Meiping Z, Hong Y et al (2014) Biosorption of Cu2+, Pb2+ and Cr6+ by a novel exopolysaccharide from Arthrobacter ps-5. Carbohydr Polym 101:50–56. https://doi.org/10.1016/j.carbpol.2013.09.021
Braissant O, Decho AW, Przekop KM et al (2009) Characteristics and turnover of exopolymeric substances in a hypersaline microbial mat. FEMS Microbiol Ecol 67:293–307. https://doi.org/10.1111/j.1574-6941.2008.00614.x
Kanamarlapudi SLRK, Muddada S (2017) Characterization of exopolysaccharide produced by Streptococcus thermophilus CC30. Biomed Res Int 2017. https://doi.org/10.1155/2017/4201809
Rajoka MSR, Mehwish HM, Hayat HF et al (2019) Correction to: Characterization, the antioxidant and antimicrobial activity of exopolysaccharide isolated from poultry origin lactobacilli (probiotics and antimicrobial proteins, (2018). Probiotics Antimicrob Proteins 11:1143–1144. https://doi.org/10.1007/s12602-018-9504-x
Liu Z, Zhang Z, Qiu L et al (2017) Characterization and bioactivities of the exopolysaccharide from a probiotic strain of Lactobacillus plantarum WLPL04. J Dairy Sci 100:6895–6905. https://doi.org/10.3168/jds.2016-11944
Wang Y, Li C, Liu P et al (2010) Physical characterization of exopolysaccharide produced by Lactobacillus plantarum KF5 isolated from Tibet Kefir. Carbohydr Polym 82:895–903. https://doi.org/10.1016/j.carbpol.2010.06.013
Saravanan C, Shetty PKH (2016) Isolation and characterization of exopolysaccharide from Leuconostoc lactis KC117496 isolated from idli batter. Int J Biol Macromol 90:100–106. https://doi.org/10.1016/j.ijbiomac.2015.02.007
Mosharaf MK, Tanvir MZH, Haque MM et al (2018) Metal-adapted bacteria isolated from wastewaters produce biofilms by expressing proteinaceous curli fimbriae and cellulose nanofibers. Front Microbiol 9:1–17. https://doi.org/10.3389/fmicb.2018.01334
Krishnamurthy M, JayaramanUthaya C, Thangavel M et al (2020) Optimization, compositional analysis, and characterization of exopolysaccharides produced by multi-metal resistant Bacillus cereus KMS3–1. Carbohydr Polym. 227:115369. https://doi.org/10.1016/j.carbpol.2019.115369
Chowdhury SR, Basak RK, Sen R, Adhikari B (2011) Characterization and emulsifying property of a carbohydrate polymer produced by Bacillus pumilus UW-02 isolated from waste water irrigated agricultural soil. Int J Biol Macromol. 48:705–712. https://doi.org/10.1016/j.ijbiomac.2011.02.019
Wang B, Song Q, Zhao F et al (2019) Purification and characterization of dextran produced by Leuconostoc pseudomesenteroides PC as a potential exopolysaccharide suitable for food applications. Process Biochem 87:187–195. https://doi.org/10.1016/j.procbio.2019.08.020
Miao M, Ma Y, Huang C et al (2015) Physicochemical properties of a water soluble extracellular homopolysaccharide from Lactobacillus reuteri SK24.003. Carbohydr PolymS 131:377–383. https://doi.org/10.1016/j.carbpol.2015.05.066
Sharma K, Sharma N, Handa S, Pathania S (2020) Purification and characterization of novel exopolysaccharides produced from Lactobacillus paraplantarum KM1 isolated from human milk and its cytotoxicity. J Genet Eng Biotechnol 18. https://doi.org/10.1186/s43141-020-00063-5
Monteagudo-Mera A, Rastall RA, Gibson GR et al (2019) Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl Microbiol Biotechnol 103:6463–6472. https://doi.org/10.1007/s00253-019-09978-7
Li W, Ji J, Chen X et al (2014) Structural elucidation and antioxidant activities of exopolysaccharides from Lactobacillus helveticus MB2–1. Carbohydr Polym 102:351–359. https://doi.org/10.1016/j.carbpol.2013.11.053
Li L, Thakur K, Liao BY, et al (2018) Antioxidant and antimicrobial potential of polysaccharides sequentially extracted from Polygonatum cyrtonema Hua. Elsevier B.V
Yu X, Zhang G, Xie C et al (2011) Equilibrium, kinetic, and thermodynamic studies of hazardous dye neutral red biosorption by spent corncob substrate. BioResources 6:936–949
Wei W, Wang Q, Li A, et al (2016) Biosorption of Pb (II) from aqueous solution by extracellular polymeric substances extracted from Klebsiella sp. J1: adsorption behavior and mechanism assessment. Sci Rep. 6:1–10. https://doi.org/10.1038/srep31575
Öztürk A, Artan T, Ayar A (2004) Biosorption of nickel(II) and copper(II) ions from aqueous solution by Streptomyces coelicolor A3(2). Colloids Surfaces B Biointerfaces. 34:105–111. https://doi.org/10.1016/j.colsurfb.2003.11.008
Zhang Z, Cai R, Zhang W, et al (2017) A novel exopolysaccharide with metal adsorption capacity produced by a marine bacterium Alteromonas sp. JL2810. Mar Drugs 15. https://doi.org/10.3390/md15060175
Petrović M, Šoštarić T, Stojanović M et al (2017) Mechanism of adsorption of Cu2+ and Zn2+ on the corn silk (Zea mays L.). Ecol Eng. 99:83–90. https://doi.org/10.1016/j.ecoleng.2016.11.057
Reddy DHK, Lee SM, Seshaiah K (2012) Biosorption of toxic heavy metal ions from water environment using honeycomb biomass-an industrial waste material. Water Air Soil Pollut 223:5967–5982. https://doi.org/10.1007/s11270-012-1332-0
Zafar MN, Aslam I, Nadeem R et al (2015) Characterization of chemically modified biosorbents from rice bran for biosorption of Ni(II). J Taiwan Inst Chem Eng 46:82–88. https://doi.org/10.1016/j.jtice.2014.08.034
Malkoc E, Nuhoglu Y (2007) Determination of kinetic and equilibrium parameters of the batch adsorption of Cr(VI) onto waste acorn of Quercus ithaburensis. Chem Eng Process Process Intensif 46:1020–1029. https://doi.org/10.1016/j.cep.2007.05.007
Gupta P, Diwan B (2017) Bacterial exopolysaccharide mediated heavy metal removal: a review on biosynthesis, mechanism and remediation strategies. Biotechnol Reports 13:58–71. https://doi.org/10.1016/j.btre.2016.12.006
Lakzian A, Berenji AR, Karimi E, Razavi S (2008) Adsorption capability of lead, nickel and zinc by exopolysaccharide and dried cell of Ensifer meliloti. Asian J Chem 20:6075–6080
Cui D, Tan C, Deng H, et al (2020) Biosorption mechanism of aqueous Pb2+, Cd2+, and Ni2+ions on extracellular polymeric substances (EPS). Archaea 2020. https://doi.org/10.1155/2020/8891543
Pagliaccia B, Carretti E, Severi M et al (2022) Heavy metal biosorption by extracellular polymeric substances (EPS) recovered from anammox granular sludge. J Hazard Mater. 424:126661. https://doi.org/10.1016/j.jhazmat.2021.126661
Chug R, Mathur S, Kothari SL et al (2021) Maximizing EPS production from Pseudomonas aeruginosa and its application in Cr and Ni sequestration. Biochem Biophys Reports 26:100972. https://doi.org/10.1016/j.bbrep.2021.100972
Acknowledgements
The authors would like to thank the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant No. 451-03-68/2022-14/200026) for financial support of this work.
Funding
This work was financially supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant No. 451–03-68/2022–14/200026).
Author information
Authors and Affiliations
Contributions
M. P. and V. Lj. designed the study; M. P., V. Lj., and M. M. performed the experiments; K. N. and M. D. performed biological assays; S. C. and M. S. contributed resources. All authors have read and approved the final version of this manuscript.
Corresponding author
Ethics declarations
Ethics approval
The manuscript does not include any experiments which were carried out with human volunteers or any kind of animals.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• EPSs obtained from the probiotic strain L. reuteri B2 were investigated.
• Biological activity and stability of the novel EPS, cytotoxic and antioxidant activity of novel EPS, and adhesion capability of the probiotic strain L. reuteri B2 were investigated.
• Biosorption studies of the removal of Ni2+ ions by EPS from L. reuteri B2, from the contaminated water, were also investigated.
• EPS from L. reuteri B2 can be considered a very promising biomaterial for potential application in different industries, from pharmacy to wastewater treatments.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ljubic, V., Milosevic, M., Cvetkovic, S. et al. The new exopolysaccharide produced by the probiotic strain L. reuteri B2: extraction, biological properties, and possible application for Ni2+ ion removal from the contaminated water. Biomass Conv. Bioref. 14, 11523–11538 (2024). https://doi.org/10.1007/s13399-022-03292-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-03292-5