Abstract
The achievement of valuable utilization of lignin will greatly alleviate the energy crisis. In this study, a series of supported bimetallic catalysts (NixZn1−x/ZrO2-MgO) were prepared and their physicochemical properties were investigated by means of SEM, XRD, XPS, BET, NH3-TPD, and H2-TPR analysis. This paper explored the effects of different Ni/Zn ratios, reaction temperatures (180 ~ 240 °C), and reaction time (2, 4, 6, and 8 h) on alkali lignin catalytic hydrogenolysis. The results showed that the highest bio-oil yield gained was 65.22 wt% under the conditions of Ni0.75Zn0.25/ZrO2-MgO catalyst at 240 °C for 6 h. The component of lignin-derived bio-oils was also analyzed qualitatively and quantitatively by GC/MS and GC/FID, respectively. It was demonstrated that the synergistic effect between Ni and Zn species contributed to the prepared catalyst having a high activity and good selectivities for phenolic compounds with a yield of 13.22 wt%. In addition, formic acid was the primary hydrogen donor in the system and isopropanol was the reaction medium as well as the secondary hydrogen donor, which facilitated the reduction of external H2 supply requirements. The catalytic stability was finally tested, and results indicated that Ni0.75Zn0.25/ZrO2-MgO still retained good catalytic performance after five cycles. By optimizing the reaction conditions and proposing the possible depolymerization route, this paper will provide an experimental basis for lignin catalytic hydrogenolysis and reliable reference for valorizing industrial lignin to value-added chemicals based on non-noble metal catalysts.
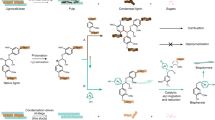
Graphical abstract













Similar content being viewed by others
Data availability
All data supporting the findings of this research are authentic and available in this article.
References
Lim CH, Lam HL (2016) Biomass supply chain optimisation via novel Biomass Element Life Cycle Analysis (BELCA). Appl Energy 161:733–745. https://doi.org/10.1016/j.apenergy.2015.07.030
Lu X, Wang D, Guo H, Xiu P, Chen J, Qin Y, Mahmud Robin H, Xu C, Zhang X, Gu X. (2021) Insights into depolymerization pathways and mechanism of alkali lignin over a Ni1.2–ZrO2/WO3/γ-Al2O3 catalyst. Chin. J. Chem. Eng. https://doi.org/10.1016/j.cjche.2021.07.018
JR Banu S Kavitha RY Kannah TP Devi M Gunasekaran SH Kim G Kumar (2019) A review on biopolymer production via lignin valorization Bioresour Technol 290. https://doi.org/10.1016/j.biortech.2019.121790
Wang Y, Sun S, Li F, Cao X, Sun R (2018) Production of vanillin from lignin: the relationship between beta-O-4 linkages and vanillin yield. Ind Crops Prod 116:116–121. https://doi.org/10.1016/j.indcrop.2018.02.043
Lu X, Wang D, Guo H, Que H, Liang D, He T, Robin HM, Xu C, Gu X (2020) Highly selective conversion from alkali lignin to phenolic products. Energy Fuels 34(11):14283–14290. https://doi.org/10.1021/acs.energyfuels.0c03098
Chio CL, Sain M, Qin WS (2019) Lignin utilization: a review of lignin depolymerization from various aspects. Renew Sust Energ Rev 107:232–249. https://doi.org/10.1016/j.rser.2019.03.008
Evstigneyev EI, Kalugina AV, Ivanov AY, Vasilyev AV (2017) Contents of β-O-4 and α-O-4 bonds in native lignin and isolated lignin preparations. J Wood Chem Technol 37(4):294–306. https://doi.org/10.1080/02773813.2017.1297832
Mei Q, Shen X, Liu H, Han B (2019) Selectively transform lignin into value-added chemicals. Chin Chem Lett 30(1):15–24. https://doi.org/10.1016/j.cclet.2018.04.032
Du B, Liu B, Wang X, Zhou J (2019) A Comparison of phenolic monomers produced from different types of lignin by phosphotungstic acid catalysts. Chemistryopen 8(5):643–649. https://doi.org/10.1002/open.201900088
Londono-Zuluaga C, Du J, Chang H-M, Jameel H, Gonzalez R (2018) Lignin Modifications and perspectives towards applications of phenolic foams: a review. Bioresources. 13(4):9158–9179. https://doi.org/10.15376/biores.13.4.Londono-Zuluaga
Lundquist K, Parkas J (2011) Different types of phenolic units in lignins. BioResources 6(2):920–926
H Ma T Li S Wu X Zhang (2020) Effect of the interaction of phenolic hydroxyl with the benzene rings on lignin pyrolysis BioresourTechnol 309. https://doi.org/10.1016/j.biortech.2020.123351
Lu X, Zhu X, Guo H, Que H, Wang D, Liang D, He T, Hu C, Xu C, Gu X (2020) Efficient depolymerization of alkaline lignin to phenolic compounds at low temperatures with formic acid over inexpensive Fe-Zn/Al2O3 catalyst. Energy Fuels 34(6):7121–7130. https://doi.org/10.1021/acs.energyfuels.0c00742
Cheng C, Li P, Yu W, Shen D, Gu S (2021) Catalytic hydrogenolysis of lignin in ethanol/isopropanol over an activated carbon supported nickel-copper catalyst. Bioresour Technol 319:124238. https://doi.org/10.1016/j.biortech.2020.124238
Taghvaei H, Moaddeli A, Khalafi-Nezhad A, Iulianelli A (2021) Catalytic hydrodeoxygenation of lignin pyrolytic-oil over Ni catalysts supported on spherical Al-MCM-41 nanoparticles: effect of Si/Al ratio and Ni loading. Fuel 293:120493. https://doi.org/10.1016/j.fuel.2021.120493
Nsimba RY, Mullen CA, West NM, Boateng AA (2013) Structure-property characteristics of pyrolytic lignins derived from fast pyrolysis of a lignin rich biomass extract. ACS Sustain Chem Eng 1(2):260–267. https://doi.org/10.1021/sc300119s
Yang X, Zhang X, Chen S (2012) Study on biopretreatment of lignin by white-rot fungi for enhancing pyrolysis in inert atmosphere. Wood Sci Technol 46(1–3):515–527. https://doi.org/10.1007/s00226-011-0420-4
Y Jia C Yang B Shen Z Ling C Huang X Li C Lai Q Yong (2021) Comparative study on enzymatic digestibility of acid-pretreated poplar and larch based on a comprehensive analysis of the lignin-derived recalcitrance Bioresour Technol 319. https://doi.org/10.1016/j.biortech.2020.124225
T Lan S Wang H Li Y Qin G Yue (2020) Effect of lignin isolated from p-toluenesulfonic acid pretreatment liquid of sugarcane bagasse on enzymatic hydrolysis of cellulose and cellulase adsorption Ind Crops Prod 155. https://doi.org/10.1016/j.indcrop.2020.112768
Yuan Y, Jiang B, Chen H, Wu W, Wu S, Jin Y, Xiao H. (2021) Recent advances in understanding the effects of lignin structural characteristics on enzymatic hydrolysis. Biotechnol. Biofuels. 14 (1). https://doi.org/10.1186/s13068-021-02054-1
M Ha Tran D-P Phan T Ha Nguyen HB Kim J Kim ED Park EY Lee (2021) Catalytic hydrogenolysis of alkali lignin in supercritical ethanol over copper monometallic catalyst supported on a chromium-based metal-organic framework for the efficient production of aromatic monomers BioresourTechnol 342. https://doi.org/10.1016/j.biortech.2021.125941
Jiang L, Guo H, Li C, Zhou P, Zhang Z (2019) Selective cleavage of lignin and lignin model compounds without external hydrogen, catalyzed by heterogeneous nickel catalysts. Chem Sci 10(16):4458–4468. https://doi.org/10.1039/c9sc00691e
Phan D-P, Lee EY (2020) Controlled hydrogenolysis over heterogeneous catalysts for lignin valorization. Catal Rev Sci Eng 62(4):607–630. https://doi.org/10.1080/01614940.2020.1770401
K Ye Y Liu S Wu J Zhuang (2021) A review for lignin valorization: challenges and perspectives in catalytic hydrogenolysisInd Crops Prod 172. https://doi.org/10.1016/j.indcrop.2021.114008
Lan W, de Bueren JB, Luterbacher JS (2019) Highly selective oxidation and depolymerization of alpha, gamma-diol-protected lignin. Angew Chem Int Ed 58(9):2649–2654. https://doi.org/10.1002/anie.201811630
Liu C, Wu S, Zhang H, Xiao R (2019) Catalytic oxidation of lignin to valuable biomass-based platform chemicals: a review. Fuel Process Technol 191:181–201. https://doi.org/10.1016/j.fuproc.2019.04.007
Liu Y, Li C, Miao W, Tang W, Xue D, Li C, Zhang B, Xiao J, Wang A, Zhang T et al (2019) Mild redox-neutral depolymerization of lignin with a binuclear Rh complex in water. Acs Catal 9(5):4441–4447. https://doi.org/10.1021/acscatal.9b00669
Hakonen KJ, Escobedo JLG, Merio-Talvio H, Hashmi SF, Karinen RS, Lehtonen J (2018) Ethanol organosolv lignin depolymerization with hydrogen over a Pd/C catalyst. ChemistrySelect 3(6):1761–1771. https://doi.org/10.1002/slct.201702701
SO Limarta H Kim J-M Ha Y-K Park J Jae (2020) High-quality and phenolic monomer-rich bio-oil production from lignin in supercritical ethanol over synergistic Ru and Mg-Zr-oxide catalysts Chem Eng J 396. https://doi.org/10.1016/j.cej.2020.125175
Dong L, Xin Y, Liu X, Guo Y, Pao C, Chen J, Wang Y (2019) Selective hydrodeoxygenation of lignin oil to valuable phenolics over Au/Nb2O5 in water. Green Chem 21(11):3081–3090. https://doi.org/10.1039/c9gc00327d
Song Y, Mobley JK, Motagamwala AH, Isaacs M, Dumesic JA, Ralph J, Lee AF, Wilson K, Crocker M (2018) Gold-catalyzed conversion of lignin to low molecular weight aromatics. Chem Sci 9(42):8127–8133. https://doi.org/10.1039/c8sc03208d
Li X, Lv Y, Pan D (2019) Pt catalysts supported on lignin-based carbon dots for methanol electro-oxidation. Colloid Surface A 569:110–118. https://doi.org/10.1016/j.colsurfa.2019.02.051
Zhang J, Su Z, Wu Z, Wang P, Xiao F (2021) Basic carrier promoted Pt-catalyzed hydrogenolysis of alkaline lignin. Catal Today 365:193–198. https://doi.org/10.1016/j.cattod.2020.06.027
MA Hossain P Thanh Khoa MS Rahaman S Tulaphol JB Jasinski N Sathitsuksanoh (2019) Catalytic cleavage of the beta-O-4 aryl ether bonds of lignin model compounds by Ru/C catalyst Appl Catal A-Gen 582. https://doi.org/10.1016/j.apcata.2019.05.034
Lin F, Ma Y, Sun Y, Zhao K, Gao T, Zhu Y (2021) Heterogeneous Ni-Ru/H-ZSM-5 one-pot catalytic conversion of lignin into monophenols. Renew Energ 170:1070–1080. https://doi.org/10.1016/j.renene.2021.01.150
Li X, Ding Y, Pan X, Xing Y, Zhang B, Liu X, Tan Y, Wang H, Li C (2022) Scission of C-O and C-C linkages in lignin over RuRe alloy catalyst. J Energy Chem 67:492–499. https://doi.org/10.1016/j.jechem.2021.10.0402095-4956
Liu Y, Li C, Miao W, Tang W, Xue D, Xiao J, Zhang T, Wang C (2020) Rhodium-terpyridine catalyzed redox-neutral depolymerization of lignin in water. Green Chem 22(1):33–38. https://doi.org/10.1039/c9gc03057c
J Gao Y Gao G Luo J Fang JH Clark S Zhang (2022) High-efficiency catalytic hydrodeoxygenation of lignin-derived vanillin with nickel-supported metal phosphate catalysts Chem Eng J 448. https://doi.org/10.1016/j.cej.2022.137723
Chanda D, Tufa R A, Aili D, Basu S. (2022) Electroreduction of CO2 to ethanol by electrochemically deposited Cu-lignin complexes on Ni foam electrodes. Nanotechnology. 33 (5). https://doi.org/10.1088/1361-6528/ac302b
Zhu J, Chen F, Zhang Z, Li M, Yang Q, Yang Y, Bao Z, Ren Q (2019) M-Gallate (M = Ni, Co) metal-organic framework-derived Ni/C and bimetallic Ni-Co/C catalysts for lignin conversion into monophenols. Acs Sustain Chem Eng 7(15):12955–12963. https://doi.org/10.1021/acssuschemeng.9b02005
Guo G, Li W, Ahmed T, Hu D, Cui R, Zhang B, Zhang X (2021) Production of liquid fuels from Kraft lignin over bimetallic Ni-Mo supported on ZIF-derived porous carbon catalyst. Rsc Adv 11(60):37932–37941. https://doi.org/10.1039/d1ra05354j
Lu X, Guo H, Wang D, Xiu P, Qin Y, Chen J, Xu C, Gu. (2021) X. A review on catalytic conversion of lignin into high-value chemicals over Ni-based catalysts. Biomass Convers. Bior. https://doi.org/10.1007/s13399-021-01903-1
H Wang Z Li H Yan Z Lei J Yan S Ren Z Wang S Kang H Shui (2022) Catalytic hydrogenolysis of lignin and model compounds over highly dispersed Ni-Ru/Al2O3 without additional H2 Fuel 326. https://doi.org/10.1016/j.fuel.2022.125027
J Guo F Liu L Bie X Si Y Li P Song N Liu Y Zhao Z Huang J Cao X Wei (2022) Selective cleavage of C-O bond in lignin and lignin model compounds over iron/nitrogen co-doped carbon supported Ni catalyst Fuel 316. https://doi.org/10.1016/j.fuel.2022.123338
Chen C, Liu P, Zhou M, Li J, Xia H, Jiang J (2021) One-step catalytic hydrotreatment of lignin dimer model compounds to cycloalkane and cycloalcohol by spherical metal- organic framework derived NiLa bimetallic materials. J Energy Inst 99:105–119. https://doi.org/10.1016/j.joei.2021.08.012
Jiang M, Chen X, Wang L, Liang J, Wei X (2021) Selective hydrogenolysis of aryl ethers over a nitrogen-doped porous carbon supported Ni-CeO2 catalyst at low temperature. Catal Sci Technol 11(9):3241–3250. https://doi.org/10.1039/dlcy00171j
Xu J, Huang Y, Yang X, He L, Zhou H, Lin Q, Zhang T, Geng H (2014) Enhancement of acetylene hydrogenation activity over Ni-Zn bimetallic catalyst by doping with Au. J Nanosci Nanotechnol 14(9):6894–6899. https://doi.org/10.1166/jnn.2014.8955
Sun H, Chen Z, Chen L, Li H, Peng Z, Liu Z, Liu S. (2018) Selective Hydrogenation of benzene to cyclohexene over Ru-Zn catalysts: investigations on the effect of Zn content and ZrO(2) as the support and dispersant. Catalysts. 8 (11). https://doi.org/10.3390/catal8110513
Raveendra G, Li C, Liu B, Cheng Y, Meng F, Li Z (2018) Synthesis of lower olefins from syngas over Zn/Al2O3-SAPO-34 hybrid catalysts: role of doped Zr and influence of the Zn/Al2O3 ratio. Catal Sci Technol 8(14):3527–3538. https://doi.org/10.1039/c8cy00574e
Xie J, Cao J, Zhao X, Jiang W, Zhao L, Zhao M, Bai H (2021) Selective cleavage of the diphenyl ether C-O bond over a Ni catalyst supported on AC with different pore structures and hydrophilicities. Energy Fuels 35(11):9599–9608. https://doi.org/10.1021/acs.energyfuels.1c00809
Song Y, Ozdemir E, Ramesh S, Adishev A, Subramanian S, Harale A, Albuali M, Fadhel BA, Jamal A, Moon D et al (2020) Dry reforming of methane by stable Ni-Mo nanocatalysts on single-crystalline MgO. Science 367(6479):777. https://doi.org/10.1126/science.aav2412
Han G-H, Lee MW, Park S, Kim HJ, Ahn J-P, Seo M-g, Lee K-Y (2019) Revealing the factors determining the selectivity of guaiacol HDO reaction pathways using ZrP-supported Co and Ni catalysts. J Catal 377:343–357. https://doi.org/10.1016/j.jcat.2019.07.034
Ma H, Li H, Zhao W, Li L, Liu S, Long J, Li X (2019) Selective depolymerization of lignin catalyzed by nickel supported on zirconium phosphate. Green Chem 21(3):658–668. https://doi.org/10.1039/c8gc03617a
Lin X, Chen L, Li H, Lv Y, Liu Y, Lu X, Liu M. (2021) Mild depolymerization of the sinocalamus oldhami alkali lignin to phenolic monomer with base and activated carbon supported nickel-tungsten carbide catalyst composite system. Bioresour. Technol. 333. https://doi.org/10.1016/j.biortech.2021.125136
Pal P, Singha RK, Saha A, Bal R, Panda AB (2015) Defect-induced efficient partial oxidation of methane over nonstoichiometric Ni/CeO2 nanocrystals. J Phys Chem C 119(24):13610–13618. https://doi.org/10.1021/acs.jpcc.5b01724
Salam MA, Cheah YW, Ho PH, Olsson L, Creaser D (2021) Hydrotreatment of lignin dimers over NiMoS-USY: effect of silica/alumina ratio. Sustain Energy Fuels 5(13):3445–3457. https://doi.org/10.1039/d1se00412c
Wei L, Bibi R, Zheng Y, Tian W, Chen L, Li N, Zhou J (2018) Promoting effect of boron on the stability and activity of Ni/Mo2C catalyst for hydrogenation of alkali lignin. Catal Lett 148(7):1856–1869. https://doi.org/10.1007/s10562-018-2395-3
Fan D, Xie X, Li Y, Li L, Sun J (2018) Aromatic compounds from lignin liquefaction over ZSM-5 catalysts in supercritical ethanol. Chem Eng Technol 41(3):509–516. https://doi.org/10.1002/ceat.201700396
Li N, Wei L, Bibi R, Chen L, Liu J, Zhang L, Zheng Y, Zhou J (2016) Catalytic hydrogenation of alkali lignin into bio-oil using flower-like hierarchical MoS2-based composite catalysts. Fuel 185:532–540. https://doi.org/10.1016/j.fuel.2016.08.001
Adilina IB, Rinaldi N, Simanungkalit SP, Aulia F, Oemry F, Stenning GBG, Silverwood IP, Parker SF (2019) Hydrodeoxygenation of guaiacol as a bio-oil model compound over pillared clay-supported nickel-molybdenum catalysts. J Phy Chem C 123(35):21429–21439. https://doi.org/10.1021/acs.jpcc.9b01890
Shu R, Zhang Q, Ma L, Xu Y, Chen P, Wang C, Wang T (2016) Insight into the solvent, temperature and time effects on the hydrogenolysis of hydrolyzed lignin. Bioresour Technol 221:568–575. https://doi.org/10.1016/j.biortech.2016.09.043
Yan B, Lin X, Chen Z, Cai Q, Zhang S. (2021) Selective production of phenolic monomers via high efficient lignin depolymerization with a carbon based nickel-iron-molybdenum carbide catalyst under mild conditions. Bioresour Technol 321. https://doi.org/10.1016/j.biortech.2020.124503
Shen X, Meng Q, Mei Q, Liu H, Yan J, Song J, Tan D, Chen B, Zhang Z, Yang G et al (2020) Selective catalytic transformation of lignin with guaiacol as the only liquid product. Chem Sci 11(5):1347–1352. https://doi.org/10.1039/c9sc05892c
Jiang Q, Sheng W, Guo X, Tang J, Guo C (2013) Metalloporphyrin-catalyzed aerobic oxidation of 2-methoxy-4-methylphenol as a route to vanillin. J Mol Catal a-Chem 373:121–126. https://doi.org/10.1016/j.molcata.2013.03.004
Sarkar MK, Kar A, Jayaraman A, Mahapatra SK, Vadivel V (2021) Pharmacokinetic properties and anti-proliferative mechanisms of vanillin against acute lymphoblastic leukemia (Jurkat) cells. S Afr J Bot 142:82–87. https://doi.org/10.1016/j.sajb.2021.06.016
Liu X, Yang J, Li J, Xu C, Jiang W (2021) Vanillin attenuates cadmium-induced lung injury through inhibition of inflammation and lung barrier dysfunction through activating AhR. Inflammation 44(6):2193–2202. https://doi.org/10.1007/s10753-021-01492-1
de Groot AC (2020) Fragrances: contact allergy and other adverse effects. Dermatitis 31(1):13–35. https://doi.org/10.1097/DER.0000000000000463
Leal Pinto SM, Rivera Y, Herrera Sandoval LV, Camilo Lizarazo J, Jairo Rincon J, Vargas Mendez LY (2019) Semisynthetic eugenol derivatives as antifungal agents against dermatophytes of the genus Trichophyton. J Med Microbiol 68(7):1109–1117. https://doi.org/10.1099/jmm.0.001019
Li J, Li B, Zhang XC (2002) Comparative studies of thermal degradation between larch lignin and manchurian ash lignin. Polym Degrad Stab 78(2):279–285. https://doi.org/10.1016/S0141-3910(02)00172-6
Sun Y, Mang JP, Yang G, Li ZH (2007) Study on the spectra of spruce lignin with chlorine dioxide oxidation. Spectrosc Spectr Anal 27(8):1551–1554
Du B, Chen C, Sun Y, Liu B, Yang Y, Si Gao, Zhang Z, Wang X, Zhou J (2020) Ni–Mg–Al catalysts effectively promote depolymerization of rice husk lignin to bio-oil. Catal. Lett. 150(6):1591–1604. https://doi.org/10.1007/s10562-019-02956-8
Ouyang X, Huang X, Zhu Y, Qiu X (2015) Ethanol-enhanced liquefaction of lignin with formic acid as an in situ hydrogen donor. Energy Fuels 29(9):5835–5840. https://doi.org/10.1021/acs.energyfuels.5b01127
Funding
This research was financially supported by the National Natural Science Foundation of China (No. 21774059).
Author information
Authors and Affiliations
Contributions
Conceptualization, Y.Q., D.W.; methodology, X.L.; software, P.X.; validation, Y.Q., J.C., and D.W.; formal analysis, X.G.; investigation, X.G.; resources, X.G.; data curation, D.W.; writing—original draft preparation, Y.Q.; writing—review and editing, X.G.; visualization, X.L.; supervision, X.G.; project administration, X.G.; funding acquisition, X.G. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
A bimetallic catalyst based on non-noble metals was developed to catalyze the depolymerization of alkaline lignin.
The introduction of Ni and Zn elements effectively improved catalytic activity and selectivity.
High yields of bio-oils (65.22 wt%) and phenolic compounds (13.22 wt%) were obtained.
Good catalytic activities were still performed after 5 recycles.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qin, Y., Wang, D., Chen, J. et al. Selective depolymerization of lignin into phenolic products over NixZn1 − x/ZrO2-MgO. Biomass Conv. Bioref. (2022). https://doi.org/10.1007/s13399-022-03254-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-022-03254-x