Abstract
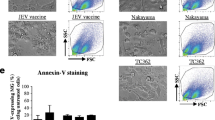
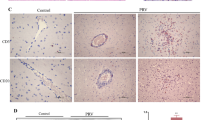
West Nile virus (WNV) has emerged as a significant cause of viral encephalitis in humans and horses. However, the pathogenesis of the West Nile encephalitis remains unclear. Microglia are activated by WNV infection, and the pathogenic involvement of their phenotypes is controversial. In this study, we examined the diversity of microglia phenotypes caused by WNV infection by assessing various microglia markers and identified disease-associated microglia in WNV-infected mouse brain tissue. Cells positive for general microglia markers such as Iba1, P2RY12, or TMEM119 were detected in the control and WNV-infected brain tissue. The morphology of the positive cells in brain tissue infected by WNV was different from that of control brain tissue, indicating that WNV infection induced activation of microglia. The activated microglia were classified into various phenotypes by investigation of specific marker expression. Among the activated microglia, disease-associated microglia that were positive for CD11c and weakly positive for TMEM119 were detected close to the WNV-infected cells. These results indicate that WNV infection induces activation of diverse microglia phenotypes and that disease-associated microglia may be associated with the pathogenicity of WNV infection in the mouse brain.




Similar content being viewed by others
Data Availability
The data is available upon request.
References
Benmamar-Badel A, Owens T, Wlodarczyk A (2020) Protective microglial subset in development, aging, and disease: lessons from transcriptomic studies. Front Immunol 11:430
Chen Z, Zhong D, Li G (2019) The role of microglia in viral encephalitis: a review. J Neuroinflammation 16:76
Chhatbar C, Prinz M (2021) The roles of microglia in viral encephalitis: from sensome to therapeutic targeting. Cell Mol Immunol 18:250–258
Clarke P, Leser JS, Quick ED, Dionne KR, Beckham JD, Tyler KL (2014) Death receptor-mediated apoptotic signaling is activated in the brain following infection with West Nile virus in the absence of a peripheral immune response. J Virol 88:1080–1089
Davis LE, DeBiasi R, Goade DE, Haaland KY, Harrington JA, Harnar JB, Pergam SA, King MK, DeMasters BK, Tyler KL (2006) West Nile virus neuroinvasive disease. Ann Neurol 60:286–300
Feng W, Zhang Y, Wang Z, Xu H, Wu T, Marshall C, Gao J, Xiao M (2020) Microglia prevent beta-amyloid plaque formation in the early stage of an Alzheimer’s disease mouse model with suppression of glymphatic clearance. Alzheimers Res Ther 12:125
Franco R, Fernández-Suárez D (2015) Alternatively activated microglia and macrophages in the central nervous system. Prog Neurobiol 131:65–86
Ghoshal A, Das S, Ghosh S, Mishra MK, Sharma V, Koli P, Sen E, Basu A (2007) Proinflammatory mediators released by activated microglia induces neuronal death in Japanese encephalitis. Glia 55:483–496
Han J, Zhu K, Zhang XM, Harris RA (2019) Enforced microglial depletion and repopulation as a promising strategy for the treatment of neurological disorders. Glia 67:217–231
Hashemiaghdam A, Mroczek M (2020) Microglia heterogeneity and neurodegeneration: the emerging paradigm of the role of immunity in Alzheimer’s disease. J Neuroimmunol 341:577185
Healy LM, Zia S, Plemel JR (2022) Towards a definition of microglia heterogeneity. Commun Biol 5:1114
Hickman S, Izzy S, Sen P, Morsett L, El Khoury J (2018) Microglia in neurodegeneration. Nat Neurosci 21:1359–1369
Jurga AM, Paleczna M, Kuter KZ (2020) Overview of general and discriminating markers of differential microglia phenotypes. Front Cell Neurosci 14:198
Kadowaki H, Nishitoh H, Urano F, Sadamitsu C, Matsuzawa A, Takeda K, Masutani H, Yodoi J, Urano Y, Nagano T, Ichijo H (2005) Amyloid beta induces neuronal cell death through ROS-mediated ASK1 activation. Cell Death Differ 12:19–24
Kenkhuis B, Somarakis A, Kleindouwel LRT, van Roon-Mom WMC, Höllt T, van der Weerd L (2022) Co-expression patterns of microglia markers Iba1, TMEM119, and P2RY12 in Alzheimer’s disease. Neurobiol Dis 167:105684
Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, Itzkovitz S, Colonna M, Schwartz M, Amit I (2017) A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169:1276-1290.e17
Kobayashi S, Orba Y, Yamaguchi H, Kimura T, Sawa H (2012) Accumulation of ubiquitinated proteins is related to West Nile virus-induced neuronal apoptosis. Neuropathology 32:398–405
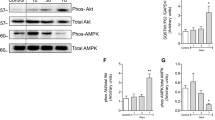
Kobayashi S, Yoshii K, Phongphaew W, Muto M, Hirano M, Orba Y, Sawa H, Kariwa H (2020) West Nile virus capsid protein inhibits autophagy by AMP-activated protein kinase degradation in neurological disease development. PLoS Pathog 16:e1008238
Konishi H, Kobayashi M, Kunisawa T, Imai K, Sayo A, Malissen B, Crocker PR, Sato K, Kiyama H (2017) Siglec-H is a microglia-specific marker that discriminates microglia from CNS-associated macrophages and CNS-infiltrating monocytes. Glia 65:1927–1943
Malmlov A, Bantle C, Aboellail T, Wagner K, Campbell CL, Eckley M, Chotiwan N, Gullberg RC, Perera R, Tjalkens R, Schountz T (2019) Experimental Zika virus infection of Jamaican fruit bats (Artibeus jamaicensis) and possible entry of virus into brain via activated microglial cells. PLoS Negl Trop Dis 13:e0007071
Orihuela R, McPherson CA, Harry GJ (2016) Microglial M1/M2 polarization and metabolic states. Br J Pharmacol 173:649–665
Peng BH, Wang T (2019) West Nile virus induced cell death in the central nervous system. Pathogens 8
Petersen LR, Brault AC, Nasci RS (2013) West Nile virus: review of the literature. JAMA 310:308–315
Quick ED, Leser JS, Clarke P, Tyler KL (2014) Activation of intrinsic immune responses and microglial phagocytosis in an ex vivo spinal cord slice culture model of West Nile virus infection. J Virol 88:13005–13014
Sato-Hashimoto M, Nozu T, Toriba R, Horikoshi A, Akaike M, Kawamoto K, Hirose A, Hayashi Y, Nagai H, Shimizu W, Saiki A, Ishikawa T, Elhanbly R, Kotani T, Murata Y, Saito Y, Naruse M, Shibasaki K, Oldenborg PA, Jung S, Matozaki T, Fukazawa Y, Ohnishi H (2019) Microglial SIRPα regulates the emergence of CD11c. Elife 8
Satoh JI, Kino Y, Yanaizu M, Ishida T, Saito Y (2019) Microglia express TMEM119 in the brains of Nasu-Hakola disease. Intractable Rare Dis Res 8:260–265
Schoch CL, Ciufo S, Domrachev M, Hotton CL, Kannan S, Khovanskaya R, Leipe D, Mcveigh R, O'Neill K, Robbertse B, Sharma S, Soussov V, Sullivan JP, Sun L, Turner S, Karsch-Mizrachi I (2020) NCBI Taxonomy: a comprehensive update on curation, resources and tools. Database (Oxford)
Seitz S, Clarke P, Tyler KL (2018) Pharmacologic depletion of microglia increases viral load in the brain and enhances mortality in murine models of flavivirus-induced encephalitis. J Virol 92
Shen X, Qiu Y, Wight AE, Kim HJ, Cantor H (2022) Definition of a mouse microglial subset that regulates neuronal development and proinflammatory responses in the brain. Proc Natl Acad Sci USA 119
Spiteri AG, Terry RL, Wishart CL, Ashhurst TM, Campbell IL, Hofer MJ, King NJC (2021) High-parameter cytometry unmasks microglial cell spatio-temporal response kinetics in severe neuroinflammatory disease. J Neuroinflammation 18:166
Stonedahl S, Clarke P, Tyler KL (2020) The role of microglia during West Nile virus infection of the central nervous system. Vaccines (Basel) 8
Stratoulias V, Venero JL, Tremblay M, Joseph B (2019) Microglial subtypes: diversity within the microglial community. EMBO J 38:e101997
Takahashi K (2023) Microglial heterogeneity in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol 82:140–149
Tan YL, Yuan Y, Tian L (2020) Microglial regional heterogeneity and its role in the brain. Mol Psychiatry 25:351–367
Todd BP, Chimenti MS, Luo Z, Ferguson PJ, Bassuk AG, Newell EA (2021) Traumatic brain injury results in unique microglial and astrocyte transcriptomes enriched for type I interferon response. J Neuroinflammation 18:151
Ulbert S (2019) West Nile virus vaccines - current situation and future directions. Hum Vaccin Immunother 15:2337–2342
Waltl I, Kalinke U (2022) Beneficial and detrimental functions of microglia during viral encephalitis. Trends Neurosci 45:158–170
Wendimu MY, Hooks SB (2022) Microglia phenotypes in aging and neurodegenerative diseases. Cells 11
Wlodarczyk A, Benmamar-Badel A, Cédile O, Jensen KN, Kramer I, Elsborg NB, Owens T (2018) CSF1R stimulation promotes increased neuroprotection by CD11c + microglia in EAE. Front Cell Neurosci 12:523
Acknowledgements
We would like to acknowledge the help of all members in the Laboratory of Public Health, Faculty of Veterinary Medicine, Hokkaido University.
Funding
This work was supported in part by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number, 20K06406; the Research Program on Emerging and Re-emerging Infectious Diseases from Japan Agency for Medical Research and Development, AMED (JP21fk0108567h0001 and JP223fa627005); Takeda Science Foundation; MSD Life Science Foundation, Public Interest Incorporated Foundation; The Akiyama Life Science Foundation; Grant for Joint Research Program of the Institute for Genetic Medicine, Hokkaido University; the cooperative research project program of the National Research Center for the Control and Prevention of Infectious Diseases, Nagasaki University; JST SPRING (JPMJSP2119); and the World-leading Innovative and Smart Education (WISE) Program (1801) from the Ministry of Education, Culture, Sports, and Technology, Japan.
Author information
Authors and Affiliations
Contributions
Conceptualization: Passawat Thammahakin and Shintaro Kobayashi; methodology: Passawat Thammahakin, Keisuke Maezono, Naoya Maekawa, and Shintaro Kobayashi; formal analysis and investigation: Passawat Thammahakin and Shintaro Kobayashi; writing—original draft preparation: Passawat Thammahakin and Shintaro Kobayashi; writing—review and editing: Hiroaki Kariwa and Shintaro Kobayashi; and supervision: Hiroaki Kariwa and Shintaro Kobayashi.
Corresponding author
Ethics declarations
Ethics statement
All animal experiments were performed following the basic guideline for animal experiments of the Ministry of Education, Culture, Sport, Science and Technology (MEXT), Japan. The President of Hokkaido University approved all animal experiments after review by the Institutional Animal Care and Use Committee of Hokkaido University (approval no. 19-0142).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thammahakin, P., Maezono, K., Maekawa, N. et al. Detection of disease-associated microglia among various microglia phenotypes induced by West Nile virus infection in mice. J. Neurovirol. 29, 367–375 (2023). https://doi.org/10.1007/s13365-023-01161-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-023-01161-z