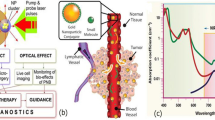
Abstract
In the present work, we have designed a one-pot green protocol in which anti-cancer drugs (curcumin and doxorubicin) can be directly loaded on the surface of gold nanoparticles during their formation. We have further demonstrated that low-intensity pulsed ultrasound (LIPUS) can be used to effectively induce the release of anti-cancer drugs from the surface of gold nanoparticles in an ex vivo tissue model. With this protocol, gold nanoparticles can be easily loaded with different types of anticancer drugs, irrespective of their affinity towards water, and even hydrophobic molecules, like curcumin, can be attached onto the gold nanoparticles in an aqueous medium. The method is very simple and straightforward and does not require stirring or mechanical shaking. The drug molecules interact with the gold seeds formed during the reduction and growth process and modulate the final morphology into a spherical shape. A black-colored colloidal solution of gold nanowire networks is formed in the absence of these anti-cancer drug molecules in the reaction mixture. We used hyperspectral-enhanced dark field microscopy to examine the uptake of gold nanoparticles by breast cancer cells. Upon exposure to LIPUS, the release of the anti-cancer drug from the particle surface can be quantified by fluorescence measurements. This release of drug molecules along with trisodium citrate from the surface of gold nanoparticles by ultrasound resulted in their destabilization and subsequent aggregation, which could be visually observed through the change in the color of colloidal sol. Cancer cell viability was studied by MTT assay to examine the efficacy of this nanoparticle-based drug delivery system. Ultraviolet-visible spectroscopy, dynamic light scattering (DLS), and transmission electron microscope (TEM) analysis were used to characterize the nanoparticles and quantify anti-cancer drug release.
Graphical abstract









Similar content being viewed by others
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on request.
References
Brenner D, Ellison L, Finley C, Fitzgerald N, Poirier A, Saint-Jacques N. Canadian cancer statistics 2022: a special report on cancer prevalence. 2022.
MacDonald V. Chemotherapy: managing side effects and safe handling. Can Vet J. 2009;50:665–8.
Kashkooli FM, Rezaeian M, Soltani M. Drug delivery through nanoparticles in solid tumors: a mechanistic understanding. Nanomedicine. 2022;17:695–716.
Grodzinski P, Kircher M, Goldberg M, Gabizon A. Integrating nanotechnology into cancer care. ACS Nano. 2019;13:7370–6.
Muddineti OS, Ghosh B, Biswas S. Current trends in using polymer coated gold nanoparticles for cancer therapy. Int J Pharm. 2015;252–67.
Jakhmola A, Vecchione R, Onesto V, Gentile F, Celentano M, Netti P. Experimental and theoretical studies on sustainable synthesis of gold sol displaying dichroic effect. Nanomaterials. 2021;11:236.
Jakhmola A, Vecchione R, Onesto V, Gentile F, Profeta M, Battista E, et al. A theoretical and experimental study on L-tyrosine and citrate mediated sustainable production of near infrared absorbing twisted gold nanorods. Mater Sci Eng C. 2021;118:111515.
Jakhmola A, Vecchione R, Gentile F, Profeta M, Manikas AC, Battista E, et al. Experimental and theoretical study of biodirected green synthesis of gold nanoflowers. Mater Today Chem. 2019;14:100203.
Lopes TS, Alves GG, Pereira MR, Granjeiro JM, Leite PEC. Advances and potential application of gold nanoparticles in nanomedicine. J Cell Biochem. 2019;120:16370–8.
Li H, Pan S, Xia P, Chang Y, Fu C, Kong W, et al. Advances in the application of gold nanoparticles in bone tissue engineering. J Biol Eng. 2020;14:14.
Riley RS, Day ES. Gold nanoparticle‐mediated photothermal therapy: applications and opportunities for multimodal cancer treatment. WIREs Nanomed Nanobi. 2017;9.
Bucharskaya AB, Khlebtsov NG, Khlebtsov BN, Maslyakova GN, Navolokin NA, Genin VD, et al. Photothermal and photodynamic therapy of tumors with plasmonic nanoparticles: challenges and prospects. Materials. 2022;15:1606.
Jakhmola A, Anton N, Vandamme TF. Inorganic nanoparticles based contrast agents for X-ray computed tomography. Adv Healthc Mater. 2012;1:413–31.
D’Acunto M, Cioni P, Gabellieri E, Presciuttini G. Exploiting gold nanoparticles for diagnosis and cancer treatments. Nanotechnology. 2021;32:192001.
Cythlmmune and Aurimune: A nanomedicine platform. [Internet]. Available from https://www.cytimmune.com/#pipeline. Accessed Nov 2022.
Gad SC, Sharp KL, Montgomery C, Payne JD, Goodrich GP. Evaluation of the toxicity of intravenous delivery of auroshell particles (Gold-Silica Nanoshells). Int J Toxicol. 2012;31:584–94.
Rastinehad AR, Anastos H, Wajswol E, Winoker JS, Sfakianos JP, Doppalapudi SK, et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc Natl Acad Sci USA. 2019;116:18590–6.
Tan KF, In LLA, Vijayaraj KP. Surface functionalization of gold nanoparticles for targeting the tumor microenvironment to improve antitumor efficiency. ACS Appl Bio Mater. 2023;6:2944–81.
Goddard ZR, Marín MJ, Russell DA, Searcey M. Active targeting of gold nanoparticles as cancer therapeutics. Chem Soc Rev. 2020;49:8774–89.
Chandran PR, Thomas RT. Gold nanoparticles in cancer drug delivery. Nanotechnol Appl Tissue Eng. 2015;221–37.
Sulaiman GM, Waheeb HM, Jabir MS, Khazaal SH, Dewir YH, Naidoo Y. Hesperidin loaded on gold nanoparticles as a drug delivery system for a successful biocompatible, anti-cancer, anti-inflammatory and phagocytosis inducer model. Sci Rep. 2020;10:9362.
Thambiraj S, Shruthi S, Vijayalakshmi R, Ravi SD. Evaluation of cytotoxic activity of docetaxel loaded gold nanoparticles for lung cancer drug delivery. Cancer Treat Res Commun. 2019;21:100157.
Safwat MA, Soliman GM, Sayed D, Attia MA. Fluorouracil-loaded gold nanoparticles for the treatment of skin cancer: development, in vitro characterization, and in vivo evaluation in a mouse skin cancer xenograft model. Mol Pharm. 2018;15:2194–205.
Vodnik VV, Mojić M, Stamenović U, Otoničar M, Ajdžanović V, Maksimović-Ivanić D, et al. Development of genistein-loaded gold nanoparticles and their antitumor potential against prostate cancer cell lines. Mater Sci Eng C. 2021;124:112078.
Chaudhary A, Dwivedi C, Gupta A, Nandi CK. One pot synthesis of doxorubicin loaded gold nanoparticles for sustained drug release. RSC Adv. 2015;5:97330–4.
Paciotti GF, Zhao J, Cao S, Brodie PJ, Tamarkin L, Huhta M, et al. Synthesis and evaluation of paclitaxel-loaded gold nanoparticles for tumor-targeted drug delivery. Bioconjug Chem. 2016;27:2646–57.
Sun H, Su J, Meng Q, Yin Q, Chen L, Gu W, et al. Cancer cell membrane-coated gold nanocages with hyperthermia-triggered drug release and homotypic target inhibit growth and metastasis of breast cancer. Adv Funct Mater. 2017;27:1604300.
Mi P. Stimuli-responsive nanocarriers for drug delivery, tumor imaging, therapy and theranostics. Theranostics. 2020;10:4557–88.
Couture O, Foley J, Kassell NF, Larrat B, Aubry JF. Review of ultrasound mediated drug delivery for cancer treatment: Updates from pre-clinical studies. Transl Cancer Res. 2014;494–511.
Moradi Kashkooli F, Soltani M, Souri M. Controlled anti-cancer drug release through advanced nano-drug delivery systems: Static and dynamic targeting strategies. J Control Release. 2020;327:316–49.
Adewale OB, Davids H, Cairncross L, Roux S. Toxicological behavior of gold nanoparticles on various models: influence of physicochemical properties and other factors. Int J Toxicol. 2019;38:357–84.
Zhou C, Long M, Qin Y, Sun X, Zheng J. Luminescent gold nanoparticles with efficient renal clearance. Angew Chem Int Ed. 2011;50:3168–72.
Kashkooli FM, Jakhmola A, Hornsby TK, Tavakkoli JJ, Kolios MC. Ultrasound-mediated nano drug delivery for treating cancer: fundamental physics to future directions. J Control Release. 2023;355:552–78.
Schroeder A, Kost J, Barenholz Y. Ultrasound, liposomes, and drug delivery: principles for using ultrasound to control the release of drugs from liposomes. Chem Phys Lipids. 2009;1–16.
Gai M, Frueh J, Tao T, Petrov AV, Petrov VV, Shesterikov EV, et al. Polylactic acid nano- and microchamber arrays for encapsulation of small hydrophilic molecules featuring drug release via high intensity focused ultrasound. Nanoscale. 2017;9:7063–70.
Fernandes DA, Fernandes DD, Li Y, Wang Y, Zhang Z, Rousseau D, et al. Synthesis of stable multifunctional perfluorocarbon nanoemulsions for cancer therapy and imaging. Langmuir. 2016;32:10870–80.
Kubota T, Kurashina Y, Zhao J, Ando K, Onoe H. Ultrasound-triggered on-demand drug delivery using hydrogel microbeads with release enhancer. Mater Des. 2021;203:109580.
Rapoport N. Ultrasound-mediated micellar drug delivery. Int J Hyperth. 2012;28:374–85.
Wu S-Y, Chen CC, Tung Y-S, Olumolade OO, Konofagou EE. Effects of the microbubble shell physicochemical properties on ultrasound-mediated drug delivery to the brain. J Control Release. 2015;212:30–40.
Elhelf IAS, Albahar H, Shah U, Oto A, Cressman E, Almekkawy M. High intensity focused ultrasound: the fundamentals, clinical applications and research trends. Diagn Interv Imaging. 2018;99:349–59.
Jakhmola A, Hornsby T, Rod K, Tavakkoli J. A novel gold nanoparticles drug delivery system: design and ex vivo tissue testing. In: IEEE International Ultrasonics Symposium (IUS). IEEE; 2020. p. 1–3.
Hornsby T, Jakhmola A, Kolios MC, Tavakkoli JJ. Significance of non-thermal effects in LIPUS induced drug release from gold nanoparticle drug carriers. In: IEEE UFFC Latin America Ultrasonics Symposium (LAUS). IEEE; 2021. p. 1–4.
Zereshkian GH, Tavakkoli J, Kevin RO. Hand-held battery-operated therapeutic ultrasonic device. Canada; 2021.
Jakhmola A, Celentano M, Vecchione R, Manikas A, Battista E, Calcagno V, et al. Self-assembly of gold nanowire networks into gold foams: production, ultrastructure and applications. Inorg Chem Front. 2017;4:1033–41.
Jakhmola A, Krishnan S, Onesto V, Gentile F, Profeta M, Manikas A, et al. Sustainable synthesis and theoretical studies of polyhedral gold nanoparticles displaying high SERS activity, NIR absorption, and cellular uptake. Mater Today Chem. 2022;26:101016.
Hornsby TK, Jakhmola A, Kolios MC, Tavakkoli J. A quantitative study of thermal and non-thermal mechanisms in ultrasound-induced nano-drug delivery. Ultrasound Med Biol. 2023;49:1288–98.
Seynhaeve ALB, Amin M, Haemmerich D, van Rhoon GC, Ten Hagen TLM. Hyperthermia and smart drug delivery systems for solid tumor therapy. Adv Drug Deliv Rev. 2020;125–44.
Hornsby TK, Kashkooli FM, Jakhmola A, Kolios MC, Tavakkoli J. Multiphysics modeling of low-intensity pulsed ultrasound induced chemotherapeutic drug release from the surface of gold nanoparticles. Cancers (Basel). 2023;15:523.
Hornsby T, Kashkooli FM, Jakhmola A, Kolios MC, Tavakkoli J. Measuring drug release induced by thermal and non-thermal effects of ultrasound in a nanodrug delivery system. In: IEEE International Ultrasonics Symposium (IUS). IEEE; 2022. p. 1–4.
Fan C, Wang S, Hong JW, Bazan GC, Plaxco KW, Heeger AJ. Beyond superquenching: hyper-efficient energy transfer from conjugated polymers to gold nanoparticles. Proc Natl Acad Sci USA. 2003;100:6297–301.
Wuithschick M, Birnbaum A, Witte S, Sztucki M, Vainio U, Pinna N, et al. Turkevich in new robes: key questions answered for the most common gold nanoparticle synthesis. ACS Nano. 2015;9:7052–71.
Koster HJ, O’Toole HJ, Chiu KL, Rojalin T, Carney RP. Homogenous high enhancement surface-enhanced Raman scattering (SERS) substrates by simple hierarchical tuning of gold nanofoams. Colloids Interface Sci Commun. 2022;47:100596.
Prasad S, Dubourdieu D, Srivastava A, Kumar P, Lall R. Metal–curcumin complexes in therapeutics: an approach to enhance pharmacological effects of curcumin. Int J Mol Sci. 2021.
Moustaoui H, Movia D, Dupont N, Bouchemal N, Casale S, Djaker N, et al. Tunable design of gold(III)-doxorubicin complex-PEGylated nanocarrier. The golden doxorubicin for oncological applications. ACS Appl Mater Interfaces. 2016;8:19946–57.
Celentano M, Jakhmola A, Profeta M, Battista E, Guarnieri D, Gentile F, et al. Diffusion limited green synthesis of ultra-small gold nanoparticles at room temperature. Colloids Surf A Physicochem Eng Asp. 2018;558:548–57.
Kulkarni NS, Ghule SB, Dhole SN. A review on hydrotropic solubilization for poorly water soluble drugs: analytical application and formulation development. Res J Pharm Technol. 2019;12:3157.
Dhapte V, Mehta P. Advances in hydrotropic solutions: an updated review. St Petersbg Polytech Univ J Phys Math. 2015;1:424–35.
Curry D, Cameron A, MacDonald B, Nganou C, Scheller H, Marsh J, et al. Adsorption of doxorubicin on citrate-capped gold nanoparticles: insights into engineering potent chemotherapeutic delivery systems. Nanoscale. 2015;7:19611–9.
Wanninger S, Lorenz V, Subhan A, Edelmann FT. Metal complexes of curcumin - synthetic strategies, structures and medicinal applications. Chem Soc Rev. 2015;44:4986–5002.
Watzky MA, Finke RG. Gold Nanoparticle formation kinetics and mechanism: a critical analysis of the “redox crystallization” mechanism. ACS Omega. 2018;3:1555–63.
Jana NR, Gearheart L, Murphy CJ. Evidence for seed-mediated nucleation in the chemical reduction of gold salts to gold nanoparticles. Chem Mater. 2001;13:2313–22.
Watzky MA, Finke RG. Transition metal nanocluster formation kinetic and mechanistic studies. A new mechanism when hydrogen is the reductant: slow, continuous nucleation and fast autocatalytic surface growth. J Am Chem Soc. 1997;119:10382–400.
Mikhlin Y, Karacharov A, Likhatski M, Podlipskaya T, Zubavichus Y, Veligzhanin A, et al. Submicrometer intermediates in the citrate synthesis of gold nanoparticles: new insights into the nucleation and crystal growth mechanisms. J Colloid Interface Sci. 2011;362:330–6.
Feng B, Zhu R, Xu S, Chen Y, Di J. A sensitive LSPR sensor based on glutathione-functionalized gold nanoparticles on a substrate for the detection of Pb 2+ ions. RSC Adv. 2018;8:4049–56.
Liu M, Chao J, Deng S, Wang K, Li K, Fan C. Dark-field microscopy in imaging of plasmon resonant nanoparticles. Colloids Surf B Biointerfaces. 2014;124:111–7.
Haiss W, Thanh NTK, Aveyard J, Fernig DG. Determination of size and concentration of gold nanoparticles from UV-Vis spectra. Anal Chem. 2007;79:4215–21.
Castillo-López DN, Pal U. Green synthesis of Au nanoparticles using potato extract: stability and growth mechanism. J Nanopart Res. 2014;16:2571.
Sathaworawong A, Wanitphakdeedecha R. Nerve injury associated with high-intensity focused ultrasound: a case report. J Cosmet Dermatol. 2018;17:162–4.
May JP, Li SD. Hyperthermia-induced drug targeting. Expert Opin Drug Deliv. 2013;511–27.
Hornsby TK, Kashkooli FM, Jakhmola A, Kolios MC, Tavakkoli J. Kinetic modelling of ultrasound-triggered chemotherapeutic drug release from the surface of gold nanoparticles. Sci Rep. 2023;13:21301.
Cobbold RSC. Foundations of biomedical ultrasound. Oxford University Press; 2007. p. 45–51.
Afadzi M, Myhre OF, Yemane PT, Bjorkoy A, Torp SH, Van Wamel A, et al. Effect of acoustic radiation force on the distribution of nanoparticles in solid tumors. IEEE Trans Ultrason Ferroelectr Freq Control. 2021.
Moradi Kashkooli F, Jakhmola A, Ferrier GA, Hornsby TK, Tavakkoli J, Kolios MC. Integrating therapeutic ultrasound with nanosized drug delivery systems in the battle against cancer. Technol Cancer Res Treat. 2023;22:1–6.
Takebe H, Nakanishi Y, Hirose Y, Ochi M. Effect of low intensity pulsed ultrasound stimulation on sinus augmentation in rabbits. Clin Oral Implants Res. 2014;25:735–41.
Hayes BT, Merrick MA, Sandrey MA, Cordova ML. Three-MHz ultrasound heats deeper into the tissues than originally theorized. J Athl Train. 2004;39:230–4.
Mittelstein DR, Ye J, Schibber EF, Roychoudhury A, Martinez LT, Fekrazad MH, et al. Selective ablation of cancer cells with low intensity pulsed ultrasound. Appl Phys Lett. 2020;116:013701.
Gao PF, Lei G, Huang CZ. Dark-field microscopy: recent advances in accurate analysis and emerging applications. Anal Chem. 2021;93:4707–26.
Acknowledgements
The authors would like to thank Dr. Graham Ferrier and Kevin Liu from Toronto Metropolitan University for their technical help in the experimental design. We also thank Brandon Victorio for analyzing nanoparticle size from TEM images by ImageJ software.
Funding
This research was funded by Ontario Research Fund-Research Excellence (ORF-RE #RE02-032), and the Natural Sciences and Engineering Research Council of Canada (NSERC) Alliance grants (ALLRP 556270-20) and NSERC Discovery grants that were awarded to J. Tavakkoli (RGPIN-2022-03799) and M.C. Kolios (RGPIN-2022-04143). Partial research funding for this project was provided through a research contract from Toronto Poly Clinic Inc. (Number: 1-51-47966) awarded to J. Tavakkoli. Farshad Moradi Kashkooli is also supported by an NSERC Banting Postdoctoral Fellowship, administered by the Government of Canada.
Author information
Authors and Affiliations
Contributions
A.J., T.K.H., F.M.K., M.C.K., K.R., and J.T.: conceptualization and idea development. A.J., T.K.H., and F.M.K.: methodology, investigation. M.C.K, K.R., and J.T.: resources. A.J., T.K.H., and F.M.K.: data gathering. A.J. and T.K.H.: writing—original draft preparation. A.J., T.K.H., F.M.K., M.C.K., and J.T.: writing—review and editing. M.C.K. and J.T.: supervision and project administration. All authors have read and agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent for publication
All authors agreed with the content and give their consent to submit the manuscript for publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jakhmola, A., Hornsby, T.K., Kashkooli, F.M. et al. Green synthesis of anti-cancer drug-loaded gold nanoparticles for low-intensity pulsed ultrasound targeted drug release. Drug Deliv. and Transl. Res. (2024). https://doi.org/10.1007/s13346-024-01516-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s13346-024-01516-x