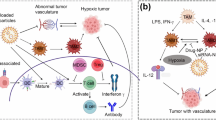
Abstract
Most of the current antitumor therapeutics were developed targeting the cancer cells only. Unfortunately, in the majority of tumors, this single-dimensional therapy is found to be ineffective. Advanced research has shown that cancer is a multicellular disorder. The tumor microenvironment (TME), which is made by a complex network of the bulk tumor cells and other supporting cells, plays a crucial role in tumor progression. Understanding the importance of the TME in tumor growth, different treatment modalities have been developed targeting these supporting cells. Recent clinical results suggest that simultaneously targeting multiple components of the tumor ecosystem with drug combinations can be highly effective. This type of “multidimensional” therapy has a high potential for cancer treatment. However, tumor-specific delivery of such multi-drug combinations remains a challenge. Nanomedicine could be utilized for the tumor-targeted delivery of such multidimensional therapeutics. In this review, we first give a brief overview of the major components of TME. We then highlight the latest developments in nanoparticle-based combination therapies, where one drug targets cancer cells and other drug targets tumor-supporting components in the TME for a synergistic effect. We include the latest preclinical and clinical studies and discuss innovative nanoparticle-mediated targeting strategies.
Graphical abstract






Similar content being viewed by others
Data availability
Not applicable.
References
Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3(6):453–8. https://doi.org/10.1038/nrc1098.
Roy A, Li SD. Modifying the tumor microenvironment using nanoparticle therapeutics. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2016;8(6):891–908. https://doi.org/10.1002/wnan.1406.
Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(17):2205–18. https://doi.org/10.1200/JCO.2012.46.3653.
Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423–37. https://doi.org/10.1038/nm.3394.
Wang M, Zhao J, Zhang L, Wei F, Lian Y, Wu Y, et al. Role of tumor microenvironment in tumorigenesis. J Cancer. 2017;8(5):761–73. https://doi.org/10.7150/jca.17648.
Son B, Lee S, Youn H, Kim E, Kim W, Youn B. The role of tumor microenvironment in therapeutic resistance. Oncotarget. 2017;8(3):3933–45. https://doi.org/10.18632/oncotarget.13907.
Li XY, Hu SQ, Xiao L. The cancer-associated fibroblasts and drug resistance. Eur Rev Med Pharmacol Sci. 2015;19(11):2112–9.
Hida K, Akiyama K, Ohga N, Maishi N, Hida Y. Tumour endothelial cells acquire drug resistance in a tumour microenvironment. J Biochem. 2013;153(3):243–9. https://doi.org/10.1093/jb/mvs152.
Tardi PG, Dos Santos N, Harasym TO, Johnstone SA, Zisman N, Tsang AW, et al. Drug ratio-dependent antitumor activity of irinotecan and cisplatin combinations in vitro and in vivo. Mol Cancer Ther. 2009;8(8):2266–75. https://doi.org/10.1158/1535-7163.MCT-09-0243.
Tardi P, Johnstone S, Harasym N, Xie S, Harasym T, Zisman N, et al. In vivo maintenance of synergistic cytarabine:daunorubicin ratios greatly enhances therapeutic efficacy. Leuk Res. 2009;33(1):129–39. https://doi.org/10.1016/j.leukres.2008.06.028.
Ma L, Kohli M, Smith A. Nanoparticles for combination drug therapy. ACS Nano. 2013;7(11):9518–25. https://doi.org/10.1021/nn405674m.
Ribeiro Franco PI, Rodrigues AP, de Menezes LB, Pacheco MM. Tumor microenvironment components: Allies of cancer progression. Pathol Res Pract. 2020;216(1):152729. https://doi.org/10.1016/j.prp.2019.152729.
Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504(7479):277–81. https://doi.org/10.1038/nature12783.
Dumont N, Liu B, Defilippis RA, Chang H, Rabban JT, Karnezis AN, et al. Breast fibroblasts modulate early dissemination, tumorigenesis, and metastasis through alteration of extracellular matrix characteristics. Neoplasia. 2013;15(3):249–62. https://doi.org/10.1593/neo.121950.
Wang FT, Sun W, Zhang JT, Fan YZ. Cancer-associated fibroblast regulation of tumor neo-angiogenesis as a therapeutic target in cancer. Oncol Lett. 2019;17(3):3055–65. https://doi.org/10.3892/ol.2019.9973.
Gascard P, Tlsty TD. Carcinoma-associated fibroblasts: orchestrating the composition of malignancy. Genes Dev. 2016;30(9):1002–19. https://doi.org/10.1101/gad.279737.116.
Martin JD, Seano G, Jain RK. Normalizing Function of Tumor Vessels: Progress, Opportunities, and Challenges. Annu Rev Physiol. 2019;81:505–34. https://doi.org/10.1146/annurev-physiol-020518-114700.
Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21(3):418–29. https://doi.org/10.1016/j.ccr.2012.01.007.
Raskov H, Orhan A, Gaggar S, Gogenur I. Cancer-associated fibroblasts and tumor-associated macrophages in cancer and cancer immunotherapy. Front Oncol. 2021;11:668731. https://doi.org/10.3389/fonc.2021.668731.
Chen X, Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discov. 2019;18(2):99–115. https://doi.org/10.1038/s41573-018-0004-1.
Jiang T, Zhang B, Zhang L, Wu X, Li H, Shen S, et al. Biomimetic nanoparticles delivered hedgehog pathway inhibitor to modify tumour microenvironment and improved chemotherapy for pancreatic carcinoma. Artif Cells Nanomed Biotechnol. 2018;46(sup1):1088–101. https://doi.org/10.1080/21691401.2018.1445093.
Albrengues J, Bourget I, Pons C, Butet V, Hofman P, Tartare-Deckert S, et al. LIF mediates proinvasive activation of stromal fibroblasts in cancer. Cell Rep. 2014;7(5):1664–78. https://doi.org/10.1016/j.celrep.2014.04.036.
Sherman MH, Yu RT, Engle DD, Ding N, Atkins AR, Tiriac H, et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159(1):80–93. https://doi.org/10.1016/j.cell.2014.08.007.
Kohli AG, Kivimae S, Tiffany MR, Szoka FC. Improving the distribution of Doxil(R) in the tumor matrix by depletion of tumor hyaluronan. J Control Release. 2014;191:105–14. https://doi.org/10.1016/j.jconrel.2014.05.019.
Chen B, Dai W, Mei D, Liu T, Li S, He B, et al. Comprehensively priming the tumor microenvironment by cancer-associated fibroblast-targeted liposomes for combined therapy with cancer cell-targeted chemotherapeutic drug delivery system. J Control Release. 2016;241:68–80. https://doi.org/10.1016/j.jconrel.2016.09.014.
Zinger A, Koren L, Adir O, Poley M, Alyan M, Yaari Z, et al. Collagenase nanoparticles enhance the penetration of drugs into pancreatic tumors. ACS Nano. 2019;13(10):11008–21. https://doi.org/10.1021/acsnano.9b02395.
Han H, Hou Y, Chen X, Zhang P, Kang M, Jin Q, et al. Metformin-induced stromal depletion to enhance the penetration of gemcitabine-loaded magnetic nanoparticles for pancreatic cancer targeted therapy. J Am Chem Soc. 2020;142(10):4944–54. https://doi.org/10.1021/jacs.0c00650.
Fang T, Zhang J, Zuo T, Wu G, Xu Y, Yang Y, et al. Chemo-photothermal combination cancer therapy with ROS Scavenging, extracellular matrix depletion, and tumor immune activation by telmisartan and diselenide-paclitaxel prodrug loaded nanoparticles. ACS Appl Mater Interfaces. 2020;12(28):31292–308. https://doi.org/10.1021/acsami.0c10416.
Li X, Qin F, Yang L, Mo L, Li L, Hou L. Sulfatide-containing lipid perfluorooctylbromide nanoparticles as paclitaxel vehicles targeting breast carcinoma. Int J Nanomedicine. 2014;9:3971–85. https://doi.org/10.2147/IJN.S67343.
Ernsting MJ, Hoang B, Lohse I, Undzys E, Cao P, Do T, et al. Targeting of metastasis-promoting tumor-associated fibroblasts and modulation of pancreatic tumor-associated stroma with a carboxymethylcellulose-docetaxel nanoparticle. J Control Release. 2015;206:122–30. https://doi.org/10.1016/j.jconrel.2015.03.023.
Murakami M, Ernsting MJ, Undzys E, Holwell N, Foltz WD, Li SD. Docetaxel conjugate nanoparticles that target alpha-smooth muscle actin-expressing stromal cells suppress breast cancer metastasis. Can Res. 2013;73(15):4862–71. https://doi.org/10.1158/0008-5472.CAN-13-0062.
Hoang B, Ernsting MJ, Roy A, Murakami M, Undzys E, Li SD. Docetaxel-carboxymethylcellulose nanoparticles target cells via a SPARC and albumin dependent mechanism. Biomaterials. 2015;59:66–76. https://doi.org/10.1016/j.biomaterials.2015.04.032.
Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002;16(21):2743–8. https://doi.org/10.1101/gad.1025302.
Cheng XB, Sato N, Kohi S, Koga A, Hirata K. 4-Methylumbelliferone inhibits enhanced hyaluronan synthesis and cell migration in pancreatic cancer cells in response to tumor-stromal interactions. Oncol Lett. 2018;15(5):6297–301. https://doi.org/10.3892/ol.2018.8147.
Mohamad Anuar NN, Nor Hisam NS, Liew SL, Ugusman A. Clinical review: navitoclax as a pro-apoptotic and anti-fibrotic agent. Front Pharmacol. 2020;11: 564108. https://doi.org/10.3389/fphar.2020.564108.
Kurelac I, Umesh Ganesh N, Iorio M, Porcelli AM, Gasparre G. The multifaceted effects of metformin on tumor microenvironment. Semin Cell Dev Biol. 2020;98:90–7. https://doi.org/10.1016/j.semcdb.2019.05.010.
Yao Y, Zou R, Liu X, Jiang J, Huang Q, He Y, et al. Telmisartan but not valsartan inhibits TGF-beta-mediated accumulation of extracellular matrix via activation of PPARgamma. J Huazhong Univ Sci Technolog Med Sci. 2008;28(5):543–8. https://doi.org/10.1007/s11596-008-0512-z.
Li S, Liquari P, McKee KK, Harrison D, Patel R, Lee S, et al. Laminin-sulfatide binding initiates basement membrane assembly and enables receptor signaling in Schwann cells and fibroblasts. J Cell Biol. 2005;169(1):179–89. https://doi.org/10.1083/jcb.200501098.
Lee J, Byun J, Shim G, Oh YK. Fibroblast activation protein activated antifibrotic peptide delivery attenuates fibrosis in mouse models of liver fibrosis. Nat Commun. 2022;13(1):1516. https://doi.org/10.1038/s41467-022-29186-8.
Kim MG, Shon Y, Kim J, Oh YK. Selective activation of anticancer chemotherapy by cancer-associated fibroblasts in the tumor microenvironment. J Natl Cancer Inst. 2017;109(1). https://doi.org/10.1093/jnci/djw186.
Lv Y, Xu C, Zhao X, Lin C, Yang X, Xin X, et al. Nanoplatform assembled from a CD44-targeted Prodrug and smart liposomes for dual targeting of tumor microenvironment and cancer cells. ACS Nano. 2018;12(2):1519–36. https://doi.org/10.1021/acsnano.7b08051.
Wu J, Akaike T, Hayashida K, Okamoto T, Okuyama A, Maeda H. Enhanced vascular permeability in solid tumor involving peroxynitrite and matrix metalloproteinases. Jpn J Cancer Res. 2001;92(4):439–51. https://doi.org/10.1111/j.1349-7006.2001.tb01114.x.
Dong X, Liu HJ, Feng HY, Yang SC, Liu XL, Lai X, et al. Enhanced Drug Delivery by Nanoscale Integration of a Nitric Oxide Donor To Induce Tumor Collagen Depletion. Nano Lett. 2019;19(2):997–1008. https://doi.org/10.1021/acs.nanolett.8b04236.
Ng HH, Shen M, Samuel CS, Schlossmann J, Bennett RG. Relaxin and extracellular matrix remodeling: Mechanisms and signaling pathways. Mol Cell Endocrinol. 2019;487:59–65. https://doi.org/10.1016/j.mce.2019.01.015.
Mardhian DF, Storm G, Bansal R, Prakash J. Nano-targeted relaxin impairs fibrosis and tumor growth in pancreatic cancer and improves the efficacy of gemcitabine in vivo. J Control Release. 2018;290:1–10. https://doi.org/10.1016/j.jconrel.2018.09.031.
Burri BJ, Edgington TS, Fair DS. Molecular interactions of the intrinsic activation complex of coagulation: binding of native and activated human factors IX and X to defined phospholipid vesicles. Biochem Biophys Acta. 1987;923(2):176–86.
Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity. 2014;40(2):274–88. https://doi.org/10.1016/j.immuni.2014.01.006.
Riabov V, Gudima A, Wang N, Mickley A, Orekhov A, Kzhyshkowska J. Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Front Physiol. 2014;5:75. https://doi.org/10.3389/fphys.2014.00075.
Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33(3):119–26. https://doi.org/10.1016/j.it.2011.12.001.
Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers. 2014;6(3):1670–90. https://doi.org/10.3390/cancers6031670.
Pathria P, Louis TL, Varner JA. Targeting tumor-associated macrophages in cancer. Trends Immunol. 2019;40(4):310–27. https://doi.org/10.1016/j.it.2019.02.003.
Larionova I, Cherdyntseva N, Liu T, Patysheva M, Rakina M, Kzhyshkowska J. Interaction of tumor-associated macrophages and cancer chemotherapy. Oncoimmunology. 2019;8(7):1596004. https://doi.org/10.1080/2162402X.2019.1596004.
Ishiwata T, Hirose T, Hirama M, Miura K, Iwakami S, Tominaga S, et al. A feasibility study of zoledronic acid combined with carboplatin/nedaplatin plus paclitaxel in patients with non-small cell lung cancer with bone metastases. Tumori. 2011;97(5):568–72. https://doi.org/10.1700/989.10713.
Sui D, Tang X, Ding J, Wang Y, Qin Y, Zhang N, et al. Sequential administration of sialic acid-modified liposomes as carriers for epirubicin and zoledronate elicit stronger antitumor effects with reduced toxicity. Int J Pharm. 2021;602:120552. https://doi.org/10.1016/j.ijpharm.2021.120552.
Luo KP, Lian YF, Zhang M, Yu H, Wang GJ, Li J. Charge convertible biomimetic micellar nanoparticles for enhanced melanoma-targeted therapy through tumor cells and tumor-associated macrophages dual chemotherapy with IDO immunotherapy. Chem Eng J. 2021;412. ARTN 128659 https://doi.org/10.1016/j.cej.2021.128659.
Sun JJ, Chen YC, Huang YX, Zhao WC, Liu YH, Venkataramanan R, et al. Programmable co-delivery of the immune checkpoint inhibitor NLG919 and chemotherapeutic doxorubicin via a redox-responsive immunostimulatory polymeric prodrug carrier. Acta Pharmacol Sin. 2017;38(6):823–34. https://doi.org/10.1038/aps.2017.44.
Genard G, Lucas S, Michiels C. Reprogramming of tumor-associated macrophages with anticancer therapies: radiotherapy versus chemo- and immunotherapies. Front Immunol. 2017;8:828. https://doi.org/10.3389/fimmu.2017.00828.
Keshavarz A, Pourbagheri-Sigaroodi A, Zafari P, Bagheri N, Ghaffari SH, Bashash D. Toll-like receptors (TLRs) in cancer; with an extensive focus on TLR agonists and antagonists. IUBMB Life. 2021;73(1):10–25. https://doi.org/10.1002/iub.2412.
Ektate K, Munteanu MC, Ashar H, Malayer J, Ranjan A. Chemo-immunotherapy of colon cancer with focused ultrasound and Salmonella-laden temperature sensitive liposomes (thermobots). Sci Rep. 2018;8(1):13062. https://doi.org/10.1038/s41598-018-30106-4.
Kuerban K, Gao X, Zhang H, Liu J, Dong M, Wu L, et al. Doxorubicin-loaded bacterial outer-membrane vesicles exert enhanced anti-tumor efficacy in non-small-cell lung cancer. Acta Pharm Sin B. 2020;10(8):1534–48. https://doi.org/10.1016/j.apsb.2020.02.002.
Roy A, Singh MS, Upadhyay P, Bhaskar S. Combined chemo-immunotherapy as a prospective strategy to combat cancer: a nanoparticle based approach. Mol Pharm. 2010;7(5):1778–88. https://doi.org/10.1021/mp100153r.
Roy A, Singh MS, Upadhyay P, Bhaskar S. Nanoparticle mediated co-delivery of paclitaxel and a TLR-4 agonist results in tumor regression and enhanced immune response in the tumor microenvironment of a mouse model. Int J Pharm. 2013;445(1–2):171–80. https://doi.org/10.1016/j.ijpharm.2013.01.045.
van der Zanden SY, Luimstra JJ, Neefjes J, Borst J, Ovaa H. Opportunities for Small molecules in cancer immunotherapy. Trends Immunol. 2020;41(6):493–511. https://doi.org/10.1016/j.it.2020.04.004.
Song C, Phuengkham H, Kim YS, Dinh VV, Lee I, Shin IW, et al. Syringeable immunotherapeutic nanogel reshapes tumor microenvironment and prevents tumor metastasis and recurrence. Nat Commun. 2019;10(1):3745. https://doi.org/10.1038/s41467-019-11730-8.
Wang X, Li B, Jing H, Dong X, Leng X. MWCNT-mediated combinatorial photothermal ablation and chemo-immunotherapy strategy for the treatment of melanoma. J Mater Chem B. 2020;8(19):4245–58. https://doi.org/10.1039/c9tb02238d.
Pawar VK, Singh Y, Sharma K, Shrivastav A, Sharma A, Singh A, et al. Doxorubicin hydrochloride loaded zymosan-polyethylenimine biopolymeric nanoparticles for dual ‘chemoimmunotherapeutic’ intervention in breast cancer. Pharm Res. 2017;34(9):1857–71. https://doi.org/10.1007/s11095-017-2195-2.
Zhang R, Wan Y, Lv H, Li F, Lee CS. DTX@VTX NPs synergy PD-L1 immune checkpoint nanoinhibitor to reshape immunosuppressive tumor microenvironment for enhancing chemo-immunotherapy. J Mater Chem B. 2021;9(36):7544–56. https://doi.org/10.1039/d1tb00269d.
Wang T, Mu W, Li F, Zhang J, Hou T, Pang X, et al. “Layer peeling” co-delivery system for enhanced RNA interference-based tumor associated macrophages-specific chemoimmunotherapy. Nanoscale. 2020;12(32):16851–63. https://doi.org/10.1039/d0nr04025h.
Hornyak L, Dobos N, Koncz G, Karanyi Z, Pall D, Szabo Z, et al. The role of indoleamine-2,3-dioxygenase in cancer development, diagnostics, and therapy. Front Immunol. 2018;9:151. https://doi.org/10.3389/fimmu.2018.00151.
Meng X, Du G, Ye L, Sun S, Liu Q, Wang H, et al. Combinatorial antitumor effects of indoleamine 2,3-dioxygenase inhibitor NLG919 and paclitaxel in a murine B16–F10 melanoma model. Int J Immunopathol Pharmacol. 2017;30(3):215–26. https://doi.org/10.1177/0394632017714696.
Campbell NK, Fitzgerald HK, Dunne A. Regulation of inflammation by the antioxidant haem oxygenase 1. Nat Rev Immunol. 2021;21(7):411–25. https://doi.org/10.1038/s41577-020-00491-x.
Yong SB, Ramishetti S, Goldsmith M, Diesendruck Y, Hazan-Halevy I, Chatterjee S, et al. Dual-targeted lipid nanotherapeutic boost for chemo-immunotherapy of cancer. Adv Mater. 2022;34(13): e2106350. https://doi.org/10.1002/adma.202106350.
Zhou Q, Liang J, Yang T, Liu J, Li B, Li Y, et al. Carfilzomib modulates tumor microenvironment to potentiate immune checkpoint therapy for cancer. EMBO Mol Med. 2022;14(1): e14502. https://doi.org/10.15252/emmm.202114502.
Cheng WT, Ho HO, Lin SY, Liu DZ, Chen LC, Sheu MT. Carfilzomib and Paclitaxel co-loaded protein nanoparticles an effective therapy against pancreatic adenocarcinomas. Int J Nanomedicine. 2021;16:6825–41. https://doi.org/10.2147/IJN.S331210.
Zhou DY, Qin J, Huang J, Wang F, Xu GP, Lv YT, et al. Zoledronic acid inhibits infiltration of tumor-associated macrophages and angiogenesis following transcatheter arterial chemoembolization in rat hepatocellular carcinoma models. Oncol Lett. 2017;14(4):4078–84. https://doi.org/10.3892/ol.2017.6717.
Bohannon JK, Hernandez A, Enkhbaatar P, Adams WL, Sherwood ER. The immunobiology of toll-like receptor 4 agonists: from endotoxin tolerance to immunoadjuvants. Shock. 2013;40(6):451–62. https://doi.org/10.1097/SHK.0000000000000042.
Lee JC, Lee EJ, Lee JH, Jun SH, Choi CW, Kim SI, et al. Klebsiella pneumoniae secretes outer membrane vesicles that induce the innate immune response. FEMS Microbiol Lett. 2012;331(1):17–24. https://doi.org/10.1111/j.1574-6968.2012.02549.x.
Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjallman AH, Ballmer-Hofer K, Schwendener RA. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Cancer. 2006;95(3):272–81. https://doi.org/10.1038/sj.bjc.6603240.
Murad YM, Clay TM. CpG oligodeoxynucleotides as TLR9 agonists: therapeutic applications in cancer. BioDrugs. 2009;23(6):361–75. https://doi.org/10.2165/11316930-000000000-00000.
de Graaff P, Berrevoets C, Rsch C, Schols HA, Verhoef K, Wichers HJ, et al. Curdlan, zymosan and a yeast-derived beta-glucan reshape tumor-associated macrophages into producers of inflammatory chemo-attractants. Cancer Immunol Immunother. 2021;70(2):547–61. https://doi.org/10.1007/s00262-020-02707-4.
Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG, et al. “Re-educating” tumor-associated macrophages by targeting NF-kappaB. J Exp Med. 2008;205(6):1261–8. https://doi.org/10.1084/jem.20080108.
Strachan DC, Ruffell B, Oei Y, Bissell MJ, Coussens LM, Pryer N, et al. CSF1R inhibition delays cervical and mammary tumor growth in murine models by attenuating the turnover of tumor-associated macrophages and enhancing infiltration by CD8(+) T cells. Oncoimmunology. 2013;2(12):e26968. https://doi.org/10.4161/onci.26968.
Shen S, Li HJ, Chen KG, Wang YC, Yang XZ, Lian ZX, et al. Spatial targeting of tumor-associated macrophages and tumor cells with a pH-sensitive cluster nanocarrier for cancer chemoimmunotherapy. Nano Lett. 2017;17(6):3822–9. https://doi.org/10.1021/acs.nanolett.7b01193.
Scheetz LM, Yu M, Li D, Castro MG, Moon JJ, Schwendeman A. Synthetic HDL nanoparticles delivering docetaxel and CpG for chemoimmunotherapy of colon adenocarcinoma. Int J Mol Sci. 2020;21(5). https://doi.org/10.3390/ijms21051777.
Roy A, Chandra S, Mamilapally S, Upadhyay P, Bhaskar S. Anticancer and immunostimulatory activity by conjugate of paclitaxel and non-toxic derivative of LPS for combined chemo-immunotherapy. Pharm Res. 2012;29(8):2294–309. https://doi.org/10.1007/s11095-012-0756-y.
Chi H, Li C, Zhao FS, Zhang L, Ng TB, Jin G, et al. Anti-tumor activity of toll-like receptor 7 agonists. Front Pharmacol. 2017;8:304. https://doi.org/10.3389/fphar.2017.00304.
Kang T, Li Y, Wang Y, Zhu J, Yang L, Huang Y, et al. Modular engineering of targeted dual-drug nanoassemblies for cancer chemoimmunotherapy. ACS Appl Mater Interfaces. 2019;11(40):36371–82. https://doi.org/10.1021/acsami.9b11881.
Prescott JA, Cook SJ. Targeting IKKbeta in cancer: challenges and opportunities for the therapeutic utilisation of IKKbeta inhibitors. Cells. 2018;7(9). https://doi.org/10.3390/cells7090115.
Wang T, Zhang J, Hou T, Yin X, Zhang N. Selective targeting of tumor cells and tumor associated macrophages separately by twin-like core-shell nanoparticles for enhanced tumor-localized chemoimmunotherapy. Nanoscale. 2019;11(29):13934–46. https://doi.org/10.1039/c9nr03374b.
Tang J, Zhang R, Guo M, Zhou H, Zhao Y, Liu Y, et al. Gd-metallofullerenol drug delivery system mediated macrophage polarization enhances the efficiency of chemotherapy. J Control Release. 2020;320:293–303. https://doi.org/10.1016/j.jconrel.2020.01.053.
Wei B, Pan J, Yuan R, Shao B, Wang Y, Guo X, et al. Polarization of tumor-associated macrophages by nanoparticle-loaded Escherichia coli combined with immunogenic cell death for cancer immunotherapy. Nano Lett. 2021;21(10):4231–40. https://doi.org/10.1021/acs.nanolett.1c00209.
Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29(6 Suppl 16):15–8. https://doi.org/10.1053/sonc.2002.37263.
Lu C, Bonome T, Li Y, Kamat AA, Han LY, Schmandt R, et al. Gene alterations identified by expression profiling in tumor-associated endothelial cells from invasive ovarian carcinoma. Cancer Res. 2007;67(4):1757–68. https://doi.org/10.1158/0008-5472.CAN-06-3700.
Hashizume H, Baluk P, Morikawa S, McLean JW, Thurston G, Roberge S, et al. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol. 2000;156(4):1363–80. https://doi.org/10.1016/S0002-9440(10)65006-7.
di Tomaso E, Capen D, Haskell A, Hart J, Logie JJ, Jain RK, et al. Mosaic tumor vessels: cellular basis and ultrastructure of focal regions lacking endothelial cell markers. Cancer Res. 2005;65(13):5740–9. https://doi.org/10.1158/0008-5472.CAN-04-4552.
De Val S, Black BL. Transcriptional control of endothelial cell development. Dev Cell. 2009;16(2):180–95. https://doi.org/10.1016/j.devcel.2009.01.014.
Nagy JA, Dvorak AM, Dvorak HF. VEGF-A and the induction of pathological angiogenesis. Annu Rev Pathol. 2007;2:251–75. https://doi.org/10.1146/annurev.pathol.2.010506.134925.
Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev. 2005;15(1):102–11. https://doi.org/10.1016/j.gde.2004.12.005.
Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, et al. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153(3):543–53. https://doi.org/10.1083/jcb.153.3.543.
Kandalaft LE, Facciabene A, Buckanovich RJ, Coukos G. Endothelin B receptor, a new target in cancer immune therapy. Clin Cancer Res. 2009;15(14):4521–8. https://doi.org/10.1158/1078-0432.CCR-08-0543.
Buckanovich RJ, Facciabene A, Kim S, Benencia F, Sasaroli D, Balint K, et al. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med. 2008;14(1):28–36. https://doi.org/10.1038/nm1699.
Wieland E, Rodriguez-Vita J, Liebler SS, Mogler C, Moll I, Herberich SE, et al. Endothelial Notch1 activity facilitates metastasis. Cancer Cell. 2017;31(3):355–67. https://doi.org/10.1016/j.ccell.2017.01.007.
Yamada K, Maishi N, Akiyama K, Towfik Alam M, Ohga N, Kawamoto T, et al. CXCL12-CXCR7 axis is important for tumor endothelial cell angiogenic property. Int J Cancer. 2015;137(12):2825–36. https://doi.org/10.1002/ijc.29655.
Zhang L, Yang N, Park JW, Katsaros D, Fracchioli S, Cao G, et al. Tumor-derived vascular endothelial growth factor up-regulates angiopoietin-2 in host endothelium and destabilizes host vasculature, supporting angiogenesis in ovarian cancer. Cancer Res. 2003;63(12):3403–12.
Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4(6):423–36. https://doi.org/10.1038/nrc1369.
Ojha T, Pathak V, Shi Y, Hennink WE, Moonen CTW, Storm G, et al. Pharmacological and physical vessel modulation strategies to improve EPR-mediated drug targeting to tumors. Adv Drug Deliv Rev. 2017;119:44–60. https://doi.org/10.1016/j.addr.2017.07.007.
Dhillon S. Bevacizumab combination therapy: a review of its use in patients with epithelial ovarian, fallopian tube, or primary peritoneal cancer. BioDrugs. 2013;27(4):375–92. https://doi.org/10.1007/s40259-013-0043-4.
Xu R, Xu C, Liu C, Cui C, Zhu J. Efficacy and safety of bevacizumab-based combination therapy for treatment of patients with metastatic colorectal cancer. Onco Targets Ther. 2018;11:8605–21. https://doi.org/10.2147/OTT.S171724.
Singh MS, Goldsmith M, Thakur K, Chatterjee S, Landesman-Milo D, Levy T, et al. An ovarian spheroid based tumor model that represents vascularized tumors and enables the investigation of nanomedicine therapeutics. Nanoscale. 2020;12(3):1894–903. https://doi.org/10.1039/c9nr09572a.
Kudo M. A new era in systemic therapy for hepatocellular carcinoma: atezolizumab plus bevacizumab combination therapy. Liver Cancer. 2020;9(2):119–37. https://doi.org/10.1159/000505189.
Blay JY, Papai Z, Tolcher AW, Italiano A, Cupissol D, Lopez-Pousa A, et al. Ombrabulin plus cisplatin versus placebo plus cisplatin in patients with advanced soft-tissue sarcomas after failure of anthracycline and ifosfamide chemotherapy: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16(5):531–40. https://doi.org/10.1016/S1470-2045(15)70102-6.
Uckun FM, Cogle CR, Lin TL, Qazi S, Trieu VN, Schiller G et al. A Phase 1B Clinical Study of combretastatin A1 diphosphate (OXi4503) and cytarabine (ARA-C) in combination (oxa) for patients with relapsed or refractory acute myeloid leukemia. Cancers (Basel). 2019;12(1). https://doi.org/10.3390/cancers12010074.
Wong PP, Demircioglu F, Ghazaly E, Alrawashdeh W, Stratford MR, Scudamore CL, et al. Dual-action combination therapy enhances angiogenesis while reducing tumor growth and spread. Cancer Cell. 2015;27(1):123–37. https://doi.org/10.1016/j.ccell.2014.10.015.
Black KL, Yin D, Ong JM, Hu J, Konda BM, Wang X, et al. PDE5 inhibitors enhance tumor permeability and efficacy of chemotherapy in a rat brain tumor model. Brain Res. 2008;1230:290–302. https://doi.org/10.1016/j.brainres.2008.06.122.
Connell JJ, Chatain G, Cornelissen B, Vallis KA, Hamilton A, Seymour L, et al. Selective permeabilization of the blood-brain barrier at sites of metastasis. J Natl Cancer Inst. 2013;105(21):1634–43. https://doi.org/10.1093/jnci/djt276.
Neijzen R, Wong MQ, Gill N, Wang H, Karim T, Anantha M, et al. Irinophore C, a lipid nanoparticulate formulation of irinotecan, improves vascular function, increases the delivery of sequentially administered 5-FU in HT-29 tumors, and controls tumor growth in patient derived xenografts of colon cancer. J Control Release. 2015;199:72–83. https://doi.org/10.1016/j.jconrel.2014.11.031.
Darge HF, Hanurry EY, Birhan YS, Mekonnen TW, Andrgie AT, Chou HY et al. Multifunctional drug-loaded micelles encapsulated in thermo-sensitive hydrogel for in vivo local cancer treatment: Synergistic effects of anti-vascular and immuno-chemotherapy. Chem Eng J. 2021;406. ARTN 126879 https://doi.org/10.1016/j.cej.2020.126879.
Sengupta S, Eavarone D, Capila I, Zhao G, Watson N, Kiziltepe T, et al. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature. 2005;436(7050):568–72. https://doi.org/10.1038/nature03794.
Verreault M, Strutt D, Masin D, Anantha M, Yung A, Kozlowski P, et al. Vascular normalization in orthotopic glioblastoma following intravenous treatment with lipid-based nanoparticulate formulations of irinotecan (Irinophore C), doxorubicin (Caelyx(R)) or vincristine. BMC Cancer. 2011;11:124. https://doi.org/10.1186/1471-2407-11-124.
Smolarczyk R, Czapla J, Jarosz-Biej M, Czerwinski K, Cichon T. Vascular disrupting agents in cancer therapy. Eur J Pharmacol. 2021;891:173692. https://doi.org/10.1016/j.ejphar.2020.173692.
Zhang C, An T, Wang D, Wan G, Zhang M, Wang H, et al. Stepwise pH-responsive nanoparticles containing charge-reversible pullulan-based shells and poly(beta-amino ester)/poly(lactic-co-glycolic acid) cores as carriers of anticancer drugs for combination therapy on hepatocellular carcinoma. J Control Release. 2016;226:193–204. https://doi.org/10.1016/j.jconrel.2016.02.030.
Hassan AY, Maulood IM, Salihi A. The vasodilatory mechanism of nitric oxide and hydrogen sulfide in the human mesenteric artery in patients with colorectal cancer. Exp Ther Med. 2021;21(3):214. https://doi.org/10.3892/etm.2021.9646.
Kang Y, Kim J, Park J, Lee YM, Saravanakumar G, Park KM, et al. Tumor vasodilation by N-Heterocyclic carbene-based nitric oxide delivery triggered by high-intensity focused ultrasound and enhanced drug homing to tumor sites for anti-cancer therapy. Biomaterials. 2019;217:119297. https://doi.org/10.1016/j.biomaterials.2019.119297.
Wei H, Wang F, Wang Y, Li T, Xiu P, Zhong J, et al. Verteporfin suppresses cell survival, angiogenesis and vasculogenic mimicry of pancreatic ductal adenocarcinoma via disrupting the YAP-TEAD complex. Cancer Sci. 2017;108(3):478–87. https://doi.org/10.1111/cas.13138.
Jiang D, Xu M, Pei Y, Huang Y, Chen Y, Ma F, et al. Core-matched nanoassemblies for targeted co-delivery of chemotherapy and photosensitizer to treat drug-resistant cancer. Acta Biomater. 2019;88:406–21. https://doi.org/10.1016/j.actbio.2019.02.009.
Guo P, Song S, Li Z, Tian Y, Zheng J, Yang X, et al. In vitro and in vivo evaluation of APRPG-modified angiogenic vessel targeting micelles for anticancer therapy. Int J Pharm. 2015;486(1–2):356–66. https://doi.org/10.1016/j.ijpharm.2015.03.067.
Wicki A, Rochlitz C, Orleth A, Ritschard R, Albrecht I, Herrmann R, et al. Targeting tumor-associated endothelial cells: anti-VEGFR2 immunoliposomes mediate tumor vessel disruption and inhibit tumor growth. Clin Cancer Res. 2012;18(2):454–64. https://doi.org/10.1158/1078-0432.CCR-11-1102.
Gao H, Yang Z, Cao S, Xiong Y, Zhang S, Pang Z, et al. Tumor cells and neovasculature dual targeting delivery for glioblastoma treatment. Biomaterials. 2014;35(7):2374–82. https://doi.org/10.1016/j.biomaterials.2013.11.076.
Zhang C, Song J, Lou L, Qi XJ, Zhao L, Fan B et al. Doxorubicin-loaded nanoparticle coated with endothelial cells-derived exosomes for immunogenic chemotherapy of glioblastoma. Bioeng Transl Med. 2020. https://doi.org/10.1002/btm2.10203.
Qu H, Li R, Liu Z, Zhang J, Luo R. Prognostic value of cancer stem cell marker CD133 expression in non-small cell lung cancer: a systematic review. Int J Clin Exp Pathol. 2013;6(11):2644–50.
Yu Z, Pestell TG, Lisanti MP, Pestell RG. Cancer stem cells. Int J Biochem Cell Biol. 2012;44(12):2144–51. https://doi.org/10.1016/j.biocel.2012.08.022.
Doherty MR, Smigiel JM, Junk DJ, Jackson MW. Cancer stem cell plasticity drives therapeutic resistance. cancers (Basel). 2016;8(1). https://doi.org/10.3390/cancers8010008.
Yiming L, Yunshan G, Bo M, Yu Z, Tao W, Gengfang L, et al. CD133 overexpression correlates with clinicopathological features of gastric cancer patients and its impact on survival: a systematic review and meta-analysis. Oncotarget. 2015;6(39):42019–27. https://doi.org/10.18632/oncotarget.5714.
Wang L, Zuo X, Xie K, Wei D. The Role of CD44 and Cancer Stem Cells. Methods Mol Biol. 2018;1692:31–42. https://doi.org/10.1007/978-1-4939-7401-6_3.
Satar NA, Fakiruddin KS, Lim MN, Mok PL, Zakaria N, Fakharuzi NA, et al. Novel triplepositive markers identified in human nonsmall cell lung cancer cell line with chemotherapy-resistant and putative cancer stem cell characteristics. Oncol Rep. 2018;40(2):669–81. https://doi.org/10.3892/or.2018.6461.
Shouval R, Furie N, Raanani P, Nagler A, Gafter-Gvili A. Autologous hematopoietic stem cell transplantation for systemic sclerosis: a systematic review and meta-analysis. Biol Blood Marrow Transplant. 2018;24(5):937–44. https://doi.org/10.1016/j.bbmt.2018.01.020.
Hu T, Zhou R, Zhao Y, Wu G. Integrin alpha6/Akt/Erk signaling is essential for human breast cancer resistance to radiotherapy. Sci Rep. 2016;6:33376. https://doi.org/10.1038/srep33376.
Lee TK, Castilho A, Cheung VC, Tang KH, Ma S, Ng IO. CD24(+) liver tumor-initiating cells drive self-renewal and tumor initiation through STAT3-mediated NANOG regulation. Cell Stem Cell. 2011;9(1):50–63. https://doi.org/10.1016/j.stem.2011.06.005.
Yamazaki H, Xu CW, Naito M, Nishida H, Okamoto T, Ghani FI, et al. Regulation of cancer stem cell properties by CD9 in human B-acute lymphoblastic leukemia. Biochem Biophys Res Commun. 2011;409(1):14–21. https://doi.org/10.1016/j.bbrc.2011.04.098.
Codony-Servat J, Rosell R. Cancer stem cells and immunoresistance: clinical implications and solutions. Transl Lung Cancer Res. 2015;4(6):689–703. https://doi.org/10.3978/j.issn.2218-6751.2015.12.11.
Suresh R, Ali S, Ahmad A, Philip PA, Sarkar FH. The Role of Cancer Stem Cells in Recurrent and Drug-Resistant Lung Cancer. Adv Exp Med Biol. 2016;890:57–74. https://doi.org/10.1007/978-3-319-24932-2_4.
Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang J, et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. 2020;5(1):8. https://doi.org/10.1038/s41392-020-0110-5.
Shen S, Xu X, Lin S, Zhang Y, Liu H, Zhang C, et al. A nanotherapeutic strategy to overcome chemotherapeutic resistance of cancer stem-like cells. Nat Nanotechnol. 2021;16(1):104–13. https://doi.org/10.1038/s41565-020-00793-0.
Confeld MI, Mamnoon B, Feng L, Jensen-Smith H, Ray P, Froberg J, et al. Targeting the tumor core: hypoxia-responsive nanoparticles for the delivery of chemotherapy to pancreatic tumors. Mol Pharm. 2020;17(8):2849–63. https://doi.org/10.1021/acs.molpharmaceut.0c00247.
Hubbard JM, Grothey A. Napabucasin: an update on the first-in-class cancer stemness inhibitor. Drugs. 2017;77(10):1091–103. https://doi.org/10.1007/s40265-017-0759-4.
Zou S, Tong Q, Liu B, Huang W, Tian Y, Fu X. Targeting STAT3 in cancer immunotherapy. Mol Cancer. 2020;19(1):145. https://doi.org/10.1186/s12943-020-01258-7.
Sulaiman A, McGarry S, El-Sahli S, Li L, Chambers J, Phan A, et al. Co-targeting bulk tumor and cscs in clinically translatable TNBC patient-derived xenografts via combination nanotherapy. Mol Cancer Ther. 2019;18(10):1755–64. https://doi.org/10.1158/1535-7163.MCT-18-0873.
Ning ST, Lee SY, Wei MF, Peng CL, Lin SY, Tsai MH, et al. Targeting colorectal cancer stem-like cells with anti-CD133 antibody-conjugated SN-38 nanoparticles. ACS Appl Mater Interfaces. 2016;8(28):17793–804. https://doi.org/10.1021/acsami.6b04403.
Wu MJ, Kim MR, Chen YS, Yang JY, Chang CJ. Retinoic acid directs breast cancer cell state changes through regulation of TET2-PKCzeta pathway. Oncogene. 2017;36(22):3193–206. https://doi.org/10.1038/onc.2016.467.
Li Y, Han Q, Zhao H, Guo Q, Zhang J. Napabucasin reduces cancer stem cell characteristics in hepatocellular carcinoma. Front Pharmacol. 2020;11:597520. https://doi.org/10.3389/fphar.2020.597520.
Qi D, Liu Y, Li J, Huang JH, Hu X, Wu E. Salinomycin as a potent anticancer stem cell agent: State of the art and future directions. Med Res Rev. 2022;42(3):1037–63. https://doi.org/10.1002/med.21870.
Wang Q, Yen YT, Xie C, Liu F, Liu Q, Wei J, et al. Combined delivery of salinomycin and docetaxel by dual-targeting gelatinase nanoparticles effectively inhibits cervical cancer cells and cancer stem cells. Drug Deliv. 2021;28(1):510–9. https://doi.org/10.1080/10717544.2021.1886378.
Al Faraj A, Shaik AS, Ratemi E, Halwani R. Combination of drug-conjugated SWCNT nanocarriers for efficient therapy of cancer stem cells in a breast cancer animal model. J Control Release. 2016;225:240–51. https://doi.org/10.1016/j.jconrel.2016.01.053.
Kim YJ, Liu Y, Li S, Rohrs J, Zhang R, Zhang X, et al. Co-eradication of breast cancer cells and cancer stem cells by cross-linked multilamellar liposomes enhances tumor treatment. Mol Pharm. 2015;12(8):2811–22. https://doi.org/10.1021/mp500754r.
Lu Y, Zhu Y, Deng S, Chen Y, Li W, Sun J et al. Targeting the sonic hedgehog pathway to suppress the expression of the cancer stem cell (CSC)-related transcription factors and CSC-driven thyroid tumor growth. Cancers (Basel). 2021;13(3). https://doi.org/10.3390/cancers13030418.
Hu K, Zhou H, Liu Y, Liu Z, Liu J, Tang J, et al. Hyaluronic acid functional amphipathic and redox-responsive polymer particles for the co-delivery of doxorubicin and cyclopamine to eradicate breast cancer cells and cancer stem cells. Nanoscale. 2015;7(18):8607–18. https://doi.org/10.1039/c5nr01084e.
Choi DS, Blanco E, Kim YS, Rodriguez AA, Zhao H, Huang TH, et al. Chloroquine eliminates cancer stem cells through deregulation of Jak2 and DNMT1. Stem Cells. 2014;32(9):2309–23. https://doi.org/10.1002/stem.1746.
Sun R, Shen S, Zhang YJ, Xu CF, Cao ZT, Wen LP, et al. Nanoparticle-facilitated autophagy inhibition promotes the efficacy of chemotherapeutics against breast cancer stem cells. Biomaterials. 2016;103:44–55. https://doi.org/10.1016/j.biomaterials.2016.06.038.
Sun R, Liu Y, Li SY, Shen S, Du XJ, Xu CF, et al. Co-delivery of all-trans-retinoic acid and doxorubicin for cancer therapy with synergistic inhibition of cancer stem cells. Biomaterials. 2015;37:405–14. https://doi.org/10.1016/j.biomaterials.2014.10.018.
Soeny K, Bogacka B, Jones B, Bouillon T. Optimizing dose regimens and fixed dose combination ratios in clinical trials. J Biopharm Stat. 2016;26(3):432–51. https://doi.org/10.1080/10543406.2015.1052478.
Wang L, Huang X, You X, Yi T, Lu B, Liu J, et al. Nanoparticle enhanced combination therapy for stem-like progenitors defined by single-cell transcriptomics in chemotherapy-resistant osteosarcoma. Signal Transduct Target Ther. 2020;5(1):196. https://doi.org/10.1038/s41392-020-00248-x.
Chitkara D, Singh S, Kumar V, Danquah M, Behrman SW, Kumar N, et al. Micellar delivery of cyclopamine and gefitinib for treating pancreatic cancer. Mol Pharm. 2012;9(8):2350–7. https://doi.org/10.1021/mp3002792.
Shin HC, Alani AW, Cho H, Bae Y, Kolesar JM, Kwon GS. A 3-in-1 polymeric micelle nanocontainer for poorly water-soluble drugs. Mol Pharm. 2011;8(4):1257–65. https://doi.org/10.1021/mp2000549.
Alfayez M, Kantarjian H, Kadia T, Ravandi-Kashani F, Daver N. CPX-351 (vyxeos) in AML. Leuk Lymphoma. 2020;61(2):288–97. https://doi.org/10.1080/10428194.2019.1660970.
Batist G, Gelmon KA, Chi KN, Miller WH Jr, Chia SK, Mayer LD, et al. Safety, pharmacokinetics, and efficacy of CPX-1 liposome injection in patients with advanced solid tumors. Clin Cancer Res. 2009;15(2):692–700. https://doi.org/10.1158/1078-0432.CCR-08-0515.
Park J, Wrzesinski SH, Stern E, Look M, Criscione J, Ragheb R, et al. Combination delivery of TGF-beta inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat Mater. 2012;11(10):895–905. https://doi.org/10.1038/nmat3355.
Tang WL, Tang WH, Szeitz A, Kulkarni J, Cullis P, Li SD. Systemic study of solvent-assisted active loading of gambogic acid into liposomes and its formulation optimization for improved delivery. Biomaterials. 2018;166:13–26. https://doi.org/10.1016/j.biomaterials.2018.03.004.
Tang WL, Tang WH, Chen WC, Diako C, Ross CF, Li SD. Development of a Rapidly Dissolvable Oral Pediatric Formulation for Mefloquine Using Liposomes. Mol Pharm. 2017;14(6):1969–79. https://doi.org/10.1021/acs.molpharmaceut.7b00077.
Tang WL, Chen WC, Roy A, Undzys E, Li SD. A simple and improved active loading method to efficiently encapsulate staurosporine into lipid-based nanoparticles for enhanced therapy of multidrug resistant cancer. Pharm Res. 2016;33(5):1104–14. https://doi.org/10.1007/s11095-015-1854-4.
Kolishetti N, Dhar S, Valencia PM, Lin LQ, Karnik R, Lippard SJ, et al. Engineering of self-assembled nanoparticle platform for precisely controlled combination drug therapy. Proc Natl Acad Sci U S A. 2010;107(42):17939–44. https://doi.org/10.1073/pnas.1011368107.
Barua S, Mitragotri S. Synergistic targeting of cell membrane, cytoplasm, and nucleus of cancer cells using rod-shaped nanoparticles. ACS Nano. 2013;7(11):9558–70. https://doi.org/10.1021/nn403913k.
Donahue ND, Acar H, Wilhelm S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv Drug Deliv Rev. 2019;143:68–96. https://doi.org/10.1016/j.addr.2019.04.008.
Feng LZ, Dong ZL, Tao DL, Zhang YC, Liu Z. The acidic tumor microenvironment: a target for smart cancer nano-theranostics. Natl Sci Rev. 2018;5(2):269–86. https://doi.org/10.1093/nsr/nwx062.
Zhang J, Li J, Shi Z, Yang Y, Xie X, Lee SM, et al. pH-sensitive polymeric nanoparticles for co-delivery of doxorubicin and curcumin to treat cancer via enhanced pro-apoptotic and anti-angiogenic activities. Acta Biomater. 2017;58:349–64. https://doi.org/10.1016/j.actbio.2017.04.029.
Swetha KL, Maravajjala K, Sharma S, Chowdhury R, Roy A. Development of a tumor extracellular pH-responsive nanocarrier by terminal histidine conjugation in a star shaped poly(lactic-co-glycolic acid). Eur Polym J. 2021;147. ARTN 110337 https://doi.org/10.1016/j.eurpolymj.2021.110337.
Ling D, Park W, Park SJ, Lu Y, Kim KS, Hackett MJ, et al. Multifunctional tumor pH-sensitive self-assembled nanoparticles for bimodal imaging and treatment of resistant heterogeneous tumors. J Am Chem Soc. 2014;136(15):5647–55. https://doi.org/10.1021/ja4108287.
Zhang Y, Yang C, Wang W, Liu J, Liu Q, Huang F, et al. Co-delivery of doxorubicin and curcumin by pH-sensitive prodrug nanoparticle for combination therapy of cancer. Sci Rep. 2016;6:21225. https://doi.org/10.1038/srep21225.
Taleb M, Ding Y, Wang B, Yang N, Han X, Du C, et al. Dopamine delivery via pH-sensitive nanoparticles for tumor blood vessel normalization and an improved effect of cancer chemotherapeutic drugs. Adv Healthc Mater. 2019;8(18):e1900283. https://doi.org/10.1002/adhm.201900283.
Wang X, Xu J, Xu X, Fang Q, Tang R. pH-sensitive bromelain nanoparticles by ortho ester crosslinkage for enhanced doxorubicin penetration in solid tumor. Mater Sci Eng C Mater Biol Appl. 2020;113:111004. https://doi.org/10.1016/j.msec.2020.111004.
Dong Z, Feng L, Zhu W, Sun X, Gao M, Zhao H, et al. CaCO3 nanoparticles as an ultra-sensitive tumor-pH-responsive nanoplatform enabling real-time drug release monitoring and cancer combination therapy. Biomaterials. 2016;110:60–70. https://doi.org/10.1016/j.biomaterials.2016.09.025.
Paliwal SR, Paliwal R, Vyas SP. A review of mechanistic insight and application of pH-sensitive liposomes in drug delivery. Drug Deliv. 2015;22(3):231–42. https://doi.org/10.3109/10717544.2014.882469.
Roux E, Lafleur M, Lataste E, Moreau P, Leroux JC. On the characterization of pH-sensitive liposome/polymer complexes. Biomacromol. 2003;4(2):240–8. https://doi.org/10.1021/bm025651x.
Xu H, Li Y, Paxton JW, Wu Z. Co-Delivery Using pH-Sensitive Liposomes to Pancreatic Cancer Cells: the Effects of Curcumin on Cellular Concentration and Pharmacokinetics of Gemcitabine. Pharm Res. 2021;38(7):1209–19. https://doi.org/10.1007/s11095-021-03072-2.
Kanamala M, Palmer BD, Jamieson SM, Wilson WR, Wu Z. Dual pH-sensitive liposomes with low pH-triggered sheddable PEG for enhanced tumor-targeted drug delivery. Nanomedicine (Lond). 2019;14(15):1971–89. https://doi.org/10.2217/nnm-2018-0510.
Fang Y, Xue J, Gao S, Lu A, Yang D, Jiang H, et al. Cleavable PEGylation: a strategy for overcoming the “PEG dilemma” in efficient drug delivery. Drug Deliv. 2017;24(sup1):22–32. https://doi.org/10.1080/10717544.2017.1388451.
Guo X, Cheng Y, Zhao X, Luo Y, Chen J, Yuan WE. Advances in redox-responsive drug delivery systems of tumor microenvironment. J Nanobiotechnology. 2018;16(1):74. https://doi.org/10.1186/s12951-018-0398-2.
Lu B, Xiao F, Wang Z, Wang B, Pan Z, Zhao W, et al. Redox-sensitive hyaluronic acid polymer prodrug nanoparticles for enhancing intracellular drug self-delivery and targeted cancer therapy. ACS Biomater Sci Eng. 2020;6(7):4106–15. https://doi.org/10.1021/acsbiomaterials.0c00762.
Bai S, Ma X, Shi X, Shao J, Zhang T, Wang Y, et al. Smart unimolecular micelle-based polyprodrug with dual-redox stimuli response for tumor microenvironment: enhanced in vivo delivery efficiency and tumor penetration. ACS Appl Mater Interfaces. 2019;11(39):36130–40. https://doi.org/10.1021/acsami.9b13214.
Wang X, Lin W, Zhang W, Li C, Sun T, Chen G, et al. Amphiphilic redox-sensitive NIR BODIPY nanoparticles for dual-mode imaging and photothermal therapy. J Colloid Interface Sci. 2019;536:208–14. https://doi.org/10.1016/j.jcis.2018.10.051.
Petrelli A, Borsali R, Fort S, Halila S. Redox tunable delivery systems: sweet block copolymer micelles via thiol-(bromo)maleimide conjugation. Chem Commun (Camb). 2016;52(82):12202–5. https://doi.org/10.1039/c6cc07136h.
Nguyen CT, Tran TH, Amiji M, Lu X, Kasi RM. Redox-sensitive nanoparticles from amphiphilic cholesterol-based block copolymers for enhanced tumor intracellular release of doxorubicin. Nanomedicine. 2015;11(8):2071–82. https://doi.org/10.1016/j.nano.2015.06.011.
Zhang L, Liu W, Lin L, Chen D, Stenzel MH. Degradable disulfide core-cross-linked micelles as a drug delivery system prepared from vinyl functionalized nucleosides via the RAFT process. Biomacromol. 2008;9(11):3321–31. https://doi.org/10.1021/bm800867n.
Fang Y, Lin X, Jin X, Yang D, Gao S, Shi K, et al. Design and fabrication of dual redox responsive nanoparticles with diselenide linkage combined photodynamically to effectively enhance gene expression. Int J Nanomedicine. 2020;15:7297–314. https://doi.org/10.2147/IJN.S266514.
Baldwin AD, Kiick KL. Reversible maleimide-thiol adducts yield glutathione-sensitive poly(ethylene glycol)-heparin hydrogels. Polym Chem. 2013;4(1):133–43. https://doi.org/10.1039/C2PY20576A.
Wu D, Fan YY, Yan HH, Li DD, Zhao Z, Chen XQ et al. Oxidation-sensitive polymeric nanocarrier-mediated cascade PDT chemotherapy for synergistic cancer therapy and potentiated checkpoint blockade immunotherapy. Chem Eng J. 2021;404. ARTN 126481 https://doi.org/10.1016/j.cej.2020.126481.
Rajendrakumar SK, Venu A, Revuri V, George Thomas R, Thirunavukkarasu GK, Zhang J, et al. Hyaluronan-stabilized redox-sensitive nanoassembly for chemo-gene therapy and dual T1/T2 MR Imaging in drug-resistant breast cancer cells. Mol Pharm. 2019;16(5):2226–34. https://doi.org/10.1021/acs.molpharmaceut.9b00189.
Xu F, Zhong H, Chang Y, Li D, Jin H, Zhang M, et al. Targeting death receptors for drug-resistant cancer therapy: Codelivery of pTRAIL and monensin using dual-targeting and stimuli-responsive self-assembling nanocomposites. Biomaterials. 2018;158:56–73. https://doi.org/10.1016/j.biomaterials.2017.12.018.
Kang Y, Lu L, Lan J, Ding Y, Yang J, Zhang Y, et al. Redox-responsive polymeric micelles formed by conjugating gambogic acid with bioreducible poly(amido amine)s for the co-delivery of docetaxel and MMP-9 shRNA. Acta Biomater. 2018;68:137–53. https://doi.org/10.1016/j.actbio.2017.12.028.
Yuan CS, Deng ZW, Qin D, Mu YZ, Chen XG, Liu Y. Hypoxia-modulatory nanomaterials to relieve tumor hypoxic microenvironment and enhance immunotherapy: Where do we stand? Acta Biomater. 2021;125:1–28. https://doi.org/10.1016/j.actbio.2021.02.030.
Kumari R, Sunil D, Ningthoujam RS. Hypoxia-responsive nanoparticle based drug delivery systems in cancer therapy: An up-to-date review. J Control Release. 2020;319:135–56. https://doi.org/10.1016/j.jconrel.2019.12.041.
Thambi T, Deepagan VG, Yoon HY, Han HS, Kim SH, Son S, et al. Hypoxia-responsive polymeric nanoparticles for tumor-targeted drug delivery. Biomaterials. 2014;35(5):1735–43. https://doi.org/10.1016/j.biomaterials.2013.11.022.
Khatoon S, Han HS, Jeon J, Rao NV, Jeong DW, Ikram M et al. Hypoxia-Responsive Mesoporous Nanoparticles for Doxorubicin Delivery. Polymers (Basel). 2018;10(4). https://doi.org/10.3390/polym10040390.
Kulkarni P, Haldar MK, You S, Choi Y, Mallik S. Hypoxia-Responsive Polymersomes for Drug Delivery to Hypoxic Pancreatic Cancer Cells. Biomacromol. 2016;17(8):2507–13. https://doi.org/10.1021/acs.biomac.6b00350.
Li M, Zhao G, Su WK, Shuai Q. Enzyme-Responsive Nanoparticles for Anti-tumor Drug Delivery. Front Chem. 2020;8:647. https://doi.org/10.3389/fchem.2020.00647.
Xu CF, Yu YL, Sun Y, Kong L, Yang CL, Hu M et al. Transformable Nanoparticle-Enabled Synergistic Elicitation and Promotion of Immunogenic Cell Death for Triple-Negative Breast Cancer Immunotherapy. Adv Funct Mater. 2019;29(45). ARTN 1905213 https://doi.org/10.1002/adfm.201905213.
Mi P. Stimuli-responsive nanocarriers for drug delivery, tumor imaging, therapy and theranostics. Theranostics. 2020;10(10):4557–88. https://doi.org/10.7150/thno.38069.
Horsman MR. Tissue physiology and the response to heat. Int J Hyperthermia. 2006;22(3):197–203. https://doi.org/10.1080/02656730600689066.
Pereira Gomes I, Aparecida Duarte J, Chaves Maia AL, Rubello D, Townsend DM, Branco de Barros AL et al. Thermosensitive Nanosystems Associated with Hyperthermia for Cancer Treatment. Pharmaceuticals (Basel). 2019;12(4). https://doi.org/10.3390/ph12040171.
Zarrintaj P, Jouyandeh M, Ganjali MR, Hadavand BS, Mozafari M, Sheiko SS, et al. Thermo-sensitive polymers in medicine: A review. Eur Polym J. 2019;117:402–23. https://doi.org/10.1016/j.eurpolymj.2019.05.024.
Sun S, Sun S, Sun Y, Wang P, Zhang J, Du W, et al. Bubble-Manipulated Local Drug Release from a Smart Thermosensitive Cerasome for Dual-Mode Imaging Guided Tumor Chemo-Photothermal Therapy. Theranostics. 2019;9(26):8138–54. https://doi.org/10.7150/thno.36762.
Guo F, Yu M, Wang J, Tan F, Li N. Smart IR780 Theranostic Nanocarrier for Tumor-Specific Therapy: Hyperthermia-Mediated Bubble-Generating and Folate-Targeted Liposomes. ACS Appl Mater Interfaces. 2015;7(37):20556–67. https://doi.org/10.1021/acsami.5b06552.
Zhao Y, Song Q, Yin Y, Wu T, Hu X, Gao X, et al. Immunochemotherapy mediated by thermosponge nanoparticles for synergistic anti-tumor effects. J Control Release. 2018;269:322–36. https://doi.org/10.1016/j.jconrel.2017.11.037.
Grull H, Langereis S. Hyperthermia-triggered drug delivery from temperature-sensitive liposomes using MRI-guided high intensity focused ultrasound. J Control Release. 2012;161(2):317–27. https://doi.org/10.1016/j.jconrel.2012.04.041.
Wei P, Cornel EJ, Du J. Ultrasound-responsive polymer-based drug delivery systems. Drug Deliv Transl Res. 2021;11(4):1323–39. https://doi.org/10.1007/s13346-021-00963-0.
Tharkar P, Varanasi R, Wong WSF, Jin CT, Chrzanowski W. Nano-Enhanced Drug Delivery and Therapeutic Ultrasound for Cancer Treatment and Beyond. Front Bioeng Biotechnol. 2019;7:324. https://doi.org/10.3389/fbioe.2019.00324.
Pucci C, Marino A, Sen O, De Pasquale D, Bartolucci M, Iturrioz-Rodriguez N, et al. Ultrasound-responsive nutlin-loaded nanoparticles for combined chemotherapy and piezoelectric treatment of glioblastoma cells. Acta Biomater. 2021. https://doi.org/10.1016/j.actbio.2021.04.005.
Thirunavukkarasu GK, Cherukula K, Lee H, Jeong YY, Park IK, Lee JY. Magnetic field-inducible drug-eluting nanoparticles for image-guided thermo-chemotherapy. Biomaterials. 2018;180:240–52. https://doi.org/10.1016/j.biomaterials.2018.07.028.
Schleich N, Danhier F, Preat V. Iron oxide-loaded nanotheranostics: major obstacles to in vivo studies and clinical translation. J Control Release. 2015;198:35–54. https://doi.org/10.1016/j.jconrel.2014.11.024.
Zhou XH, Wang LC, Xu YJ, Du WX, Cai XJ, Wang FJ, et al. A pH and magnetic dual-response hydrogel for synergistic chemo-magnetic hyperthermia tumor therapy. RSC Adv. 2018;8(18):9812–21. https://doi.org/10.1039/c8ra00215k.
Chen Q, Liu L, Lu Y, Chen X, Zhang Y, Zhou W, et al. Tumor Microenvironment-Triggered Aggregated Magnetic Nanoparticles for Reinforced Image-Guided Immunogenic Chemotherapy. Adv Sci (Weinh). 2019;6(6):1802134. https://doi.org/10.1002/advs.201802134.
Ahmad A, Gupta A, Ansari MM, Vyawahare A, Jayamurugan G, Khan R. Hyperbranched Polymer-Functionalized Magnetic Nanoparticle-Mediated Hyperthermia and Niclosamide Bimodal Therapy of Colorectal Cancer Cells. ACS Biomater Sci Eng. 2020;6(2):1102–11. https://doi.org/10.1021/acsbiomaterials.9b01947.
Wang C, Yang J, Luo H, Wang K, Wang Y, Xiao ZX, et al. CancerTracer: a curated database for intrapatient tumor heterogeneity. Nucleic Acids Res. 2020;48(D1):D797–806. https://doi.org/10.1093/nar/gkz1061.
Golombek SK, May JN, Theek B, Appold L, Drude N, Kiessling F, et al. Tumor targeting via EPR: Strategies to enhance patient responses. Adv Drug Deliv Rev. 2018;130:17–38. https://doi.org/10.1016/j.addr.2018.07.007.
Eberhard A, Kahlert S, Goede V, Hemmerlein B, Plate KH, Augustin HG. Heterogeneity of angiogenesis and blood vessel maturation in human tumors: implications for antiangiogenic tumor therapies. Cancer Res. 2000;60(5):1388–93.
Lammers T, Kiessling F, Hennink WE, Storm G. Drug targeting to tumors: principles, pitfalls and (pre-) clinical progress. J Control Release. 2012;161(2):175–87. https://doi.org/10.1016/j.jconrel.2011.09.063.
Achilles EG, Fernandez A, Allred EN, Kisker O, Udagawa T, Beecken WD, et al. Heterogeneity of angiogenic activity in a human liposarcoma: a proposed mechanism for “no take” of human tumors in mice. J Natl Cancer Inst. 2001;93(14):1075–81.
Yu JL, Rak JW, Carmeliet P, Nagy A, Kerbel RS, Coomber BL. Heterogeneous vascular dependence of tumor cell populations. Am J Pathol. 2001;158(4):1325–34. https://doi.org/10.1016/S0002-9440(10)64083-7.
Zhang L, Nishihara H, Kano MR. Pericyte-coverage of human tumor vasculature and nanoparticle permeability. Biol Pharm Bull. 2012;35(5):761–6. https://doi.org/10.1248/bpb.35.761.
Kano MR, Komuta Y, Iwata C, Oka M, Shirai YT, Morishita Y, et al. Comparison of the effects of the kinase inhibitors imatinib, sorafenib, and transforming growth factor-beta receptor inhibitor on extravasation of nanoparticles from neovasculature. Cancer Sci. 2009;100(1):173–80. https://doi.org/10.1111/j.1349-7006.2008.01003.x.
Gillies RJ, Schornack PA, Secomb TW, Raghunand N. Causes and effects of heterogeneous perfusion in tumors. Neoplasia. 1999;1(3):197–207. https://doi.org/10.1038/sj.neo.7900037.
Walker C, Mojares E, Del Rio Hernandez A. Role of Extracellular Matrix in Development and Cancer Progression. Int J Mol Sci. 2018;19(10). https://doi.org/10.3390/ijms19103028.
van der Meel R, Sulheim E, Shi Y, Kiessling F, Mulder WJM, Lammers T. Smart cancer nanomedicine. Nat Nanotechnol. 2019;14(11):1007–17. https://doi.org/10.1038/s41565-019-0567-y.
Pershina AG, Brikunova OY, Demin AM, Abakumov MA, Vaneev AN, Naumenko VA, et al. Variation in tumor pH affects pH-triggered delivery of peptide-modified magnetic nanoparticles. Nanomedicine. 2021;32:102317. https://doi.org/10.1016/j.nano.2020.102317.
Ho L, Bokharaei M, Li SD. Current update of a thermosensitive liposomes composed of DPPC and Brij78. J Drug Target. 2018;26(5–6):407–19. https://doi.org/10.1080/1061186X.2017.1419361.
Journe F, Chaboteaux C, Laurent G, Body JJ. Sequence-dependent synergistic effects of ibandronate in combination with antiestrogens on growth inhibition of estrogen receptor-positive breast cancer cells. Bone. 2006;38(3):S52–3. https://doi.org/10.1016/j.bone.2005.12.049.
Lu D, Wientjes MG, Lu Z, Au JL. Tumor priming enhances delivery and efficacy of nanomedicines. J Pharmacol Exp Ther. 2007;322(1):80–8. https://doi.org/10.1124/jpet.107.121632.
Raemdonck K, De Smedt SC. Lessons in simplicity that should shape the future of drug delivery. Nat Biotechnol. 2015;33(10):1026–7. https://doi.org/10.1038/nbt.3366.
Garralda E, Paz K, Lopez-Casas PP, Jones S, Katz A, Kann LM, et al. Integrated next-generation sequencing and avatar mouse models for personalized cancer treatment. Clin Cancer Res. 2014;20(9):2476–84. https://doi.org/10.1158/1078-0432.CCR-13-3047.
Ernsting MJ, Murakami M, Roy A, Li SD. Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles. J Control Release. 2013;172(3):782–94. https://doi.org/10.1016/j.jconrel.2013.09.013.
de Jong M, Maina T. Of mice and humans: are they the same?–Implications in cancer translational research. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2010;51(4):501–4. https://doi.org/10.2967/jnumed.109.065706.
De Guillebon E, Dardenne A, Saldmann A, Seguier S, Tran T, Paolini L, et al. Beyond the concept of cold and hot tumors for the development of novel predictive biomarkers and the rational design of immunotherapy combination. Int J Cancer. 2020;147(6):1509–18. https://doi.org/10.1002/ijc.32889.
Kerbel RS. Tumor angiogenesis: past, present and the near future. Carcinogenesis. 2000;21(3):505–15. https://doi.org/10.1093/carcin/21.3.505.
Friedman G, Levi-Galibov O, David E, Bornstein C, Giladi A, Dadiani M, et al. Cancer-associated fibroblast compositions change with breast cancer progression linking the ratio of S100A4(+) and PDPN(+) CAFs to clinical outcome. Nat Cancer. 2020;1(7):692–708. https://doi.org/10.1038/s43018-020-0082-y.
Jung JY, Kim HS, Roh MR, Roh HJ, Lee SY, Chung KY. The effect of imiquimod on matrix metalloproteinases and tissue inhibitors of metalloproteinases in malignant melanoma cell invasion. Ann Dermatol. 2014;26(3):363–73. https://doi.org/10.5021/ad.2014.26.3.363.
Kim D, Wu Y, Oh Y-K. Chapter Seven - Targeting cancer-associated fibroblasts in immunotherapy. In: Amiji MM, Milane LS, editors. Systemic Drug Delivery Strategies. Academic Press; 2022. p. 163–209.
Sharma M, Turaga RC, Yuan Y, Satyanarayana G, Mishra F, Bian Z et al. Simultaneously targeting cancer-associated fibroblasts and angiogenic vessel as a treatment for TNBC. J Exp Med. 2021;218(4). https://doi.org/10.1084/jem.20200712.
Hashemi Goradel N, Najafi M, Salehi E, Farhood B, Mortezaee K. Cyclooxygenase-2 in cancer: A review. J Cell Physiol. 2019;234(5):5683–99. https://doi.org/10.1002/jcp.27411.
Li S, Jiang M, Wang L, Yu S. Combined chemotherapy with cyclooxygenase-2 (COX-2) inhibitors in treating human cancers: Recent advancement. Biomed Pharmacother. 2020;129:110389. https://doi.org/10.1016/j.biopha.2020.110389.
Pu D, Yin L, Huang L, Qin C, Zhou Y, Wu Q, et al. Cyclooxygenase-2 Inhibitor: A Potential Combination Strategy With Immunotherapy in Cancer. Front Oncol. 2021;11:637504. https://doi.org/10.3389/fonc.2021.637504.
Funding
SDL and AR acknowledge funding support from Shastri Indo-Canadian Institute.
Author information
Authors and Affiliations
Contributions
Karnam Laxmi Swetha: Conceptualization; formal analysis; investigation; methodology; writing—original draft. Kavya Sree Maravajjala: Data curation; methodology; writing—original draft. Shyh-Dar Li: Funding acquisition; writing—review and editing. Manu Smriti Singh: Conceptualization; formal analysis; writing, original draft; writing, review and editing. Aniruddha Roy: Conceptualization; supervision; formal analysis; funding acquisition; writing, original draft, writing, review and editing.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Manuscript does not include any personal data.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Swetha, K.L., Maravajjala, K.S., Li, SD. et al. Breaking the niche: multidimensional nanotherapeutics for tumor microenvironment modulation. Drug Deliv. and Transl. Res. 13, 105–134 (2023). https://doi.org/10.1007/s13346-022-01194-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-022-01194-7