Abstract
We aimed to develop a simple yet novel method to prepare plasmid DNA-loaded nanoliposomes for cancer gene therapy. Murine interleukin-12 (mIL-12) pDNA-loaded nanoliposomes were prepared via novel freeze-drying of a monophase solution method. The physicochemical characteristics, cytotoxicity, and transfection efficiency of the prepared nanoliposomes in murine CT-26 colon carcinoma cells were evaluated. Furthermore, tumor progression and survival rate in CT-26 colon carcinoma-bearing BALB/c mice subsequent to direct intratumoral injections were investigated over a period of 40 days. Using this preparation method, nanoliposomes with particle size of around 300 nm and zeta potential of 96.5 mV were obtained. The transmission electron microscope results showed that the liposomes were nano-sized and almost spherical. The agarose gel retardation assay revealed the pDNA encapsulation in the nanoliposomes. The nanoliposomes with 72.4% encapsulation efficiency and low cell toxicity could significantly improve mIL-12 expression by approximately 25-fold relative to the naked mIL-12 pDNA. There was a significant tumor growth inhibition after repeated injections of mIL-12 pDNA-loaded nanoliposomes. This is the first study on the freeze-drying of a monophase solution method as a simple yet novel technique for the preparation of pDNA-loaded nanoliposomes. Given the ease of preparation method and promising in vitro and in vivo characteristics, this investigation demonstrates advances in pDNA lipid formulation for cancer gene therapy.
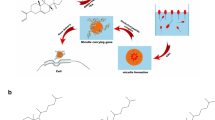
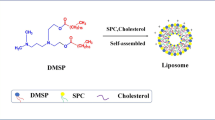
Graphical abstract









Similar content being viewed by others
Availability of data and materials
All data generated during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Chol:
-
cholesterol
- DOPE:
-
1, 2-dioleoyl-sn-glycero-3-phosphoethanolamine
- DOTAP:
-
dioleoyl trimethylammonium propane
- FBS:
-
fetal bovine serum
- mIL-12:
-
murine interleukin-12
- MTT:
-
4,5-dimethylthiazol-2-yl)-2,5 -diphenyl tetrazolium bromide
- TEM:
-
transmission electron microscopy
References
Habib N. Cancer gene therapy: past achievements and future challenges: Springer Science & Business Media. 2006.
Seth P. Vector-mediated cancer gene therapy: an overview. Cancer Biol Ther. 2005;4(5):512–7.
Hallaj-Nezhadi S, Dass CR, Lotfipour F. Intraperitoneal delivery of nanoparticles for cancer gene therapy. Future Oncol. 2013;9(1):59–68.
Gao X, Kim K-S, Liu D. Nonviral gene delivery: what we know and what is next. The AAPS J. 2007;9(1):E92–104.
Amreddy N, Babu A, Muralidharan R, Panneerselvam J, Srivastava A, Ahmed R, et al. Recent advances in nanoparticle-based cancer drug and gene delivery. Adv Cancer Res Elsevier. 2018;137:115–70.
Mei Y, Wang R, Jiang W, Bo Y, Zhang T, Yu J, et al. Recent progress in nanomaterials for nucleic acid delivery in cancer immunotherapy. Biomater Sci. 2019.
Lee H-Y, Mohammed KA, Nasreen N. Nanoparticle-based targeted gene therapy for lung cancer. Am J Cancer Res. 2016;6(5):1118.
Hallaj-Nezhadi S, Dass C, Lotfipour F. Nanoparticle-mediated interleukin-12 cancer gene therapy. J Pharm Pharm Sci. 2010;13(3):472–85.
Dass CR, Choong PF. Selective gene delivery for cancer therapy using cationic liposomes: in vivo proof of applicability. J Control Release. 2006;113(2):155–63.
Firouzabadi FB, Oryan S, Sheikhha MH, Kalantar SM, Javed A. Preparation and evaluation of a novel liposomal nano-formulation in metastatic cancer treatment studies. Cell J (Yakhteh). 2019;12(2).
Barba AA, Bochicchio S, Dalmoro A, Lamberti G. Lipid delivery systems for nucleic-acid-based-drugs: from production to clinical applications. Pharmaceutics. 2019;11(8):360.
Wang H, Liu S, Jia L, Chu F, Zhou Y, He Z, et al. Nanostructured lipid carriers for microRNA delivery in tumor gene therapy. Cancer Cell Int. 2018;18(1):101.
Chen Z, Liu F, Chen Y, Liu J, Wang X, Chen AT, et al. Targeted delivery of CRISPR/Cas9-mediated cancer gene therapy via liposome-templated hydrogel nanoparticles. Adv Funct Mater. 2017;27(46):1703036.
Wu SY, McMillan NA. Lipidic systems for in vivo siRNA delivery. The AAPS J. 2009;11(4):639–52.
Hallaj-Nezhadi S, Hassan M. Nanoliposome-based antibacterial drug delivery. Drug Deliv. 2015;22(5):581–9.
MacLachlan I. Liposomal formulations for nucleic acid delivery. Antisense drug technology: principles, strategies, and applications. 2007;2:237–70.
Wu SY, Putral LN, Liang M, Chang H-I, Davies NM, McMillan NA. Development of a novel method for formulating stable siRNA-loaded lipid particles for in vivo use. Pharmaceutical research. 2009;26(3):512.
Li C, Deng Y. A novel method for the preparation of liposomes: freeze drying of monophase solutions. Journal of pharmaceutical sciences. 2004;93(6):1403–14.
Nguyen KG, Vrabel MR, Mantooth SM, Hopkins JJ, Wagner ES, Gabaldon TA, et al. Localized interleukin-12 for cancer immunotherapy. Front Immunol. 2020;11.
Wang P, Li X, Wang J, Gao D, Li Y, Li H, et al. Re-designing Interleukin-12 to enhance its safety and potential as an anti-tumor immunotherapeutic agent. Nat Commun. 2017;8(1):1–15.
Patel A, Cholkar K, Mitra AK. Recent developments in protein and peptide parenteral delivery approaches. Ther Deliv. 2014;5(3):337–65.
Hamza T, Barnett JB, Li B. Interleukin 12 a key immunoregulatory cytokine in infection applications. Int J Mol Sci. 2010;11(3):789–806.
Shi F, Rakhmilevich AL, Heise CP, Oshikawa K, Sondel PM, Yang N-S, et al. Intratumoral injection of interleukin-12 plasmid DNA, either naked or in complex with cationic lipid, results in similar tumor regression in a murine model. Mol Cancer Ther. 2002;1(11):949–57.
Rakhmilevich AL, Timmins JG, Janssen K, Pohlmann EL, Sheehy MJ, Yang N-S. Gene gun-mediated IL-12 gene therapy induces antitumor effects in the absence of toxicity: a direct comparison with systemic IL-12 protein therapy. J Immunother (Hagerstown, Md: 1997). 1999;22(2):135–44.
Schultz J, Pavlovic J, Strack B, Nawrath M, Moelling K. Long-lasting anti-metastatic efficiency of interleukin 12-encoding plasmid DNA. Hum Gene Ther. 1999;10(3):407–17.
Puisieux I, Odin L, Poujol D, Moingeon P, Tartaglia J, Cox W, et al. Canarypox virus-mediated interleukin 12 gene transfer into murine mammary adenocarcinoma induces tumor suppression and long-term antitumoral immunity. Hum Gene Ther. 1998;9(17):2481–92.
Jhonsi MA, Ananth DA, Nambirajan G, Sivasudha T, Yamini R, Bera S, et al. Antimicrobial activity, cytotoxicity and DNA binding studies of carbon dots. Spectrochim Acta Part A Mol Biomol Spectrosc. 2018;196:295–302.
Nomura T, Yasuda K, Yamada T, Okamoto S, Mahato R, Watanabe Y, et al. Gene expression and antitumor effects following direct interferon (IFN)-γ gene transfer with naked plasmid DNA and DC-chol liposome complexes in mice. Gene Ther. 1999;6(1):121–9.
Legido M, Abell C. Comparative study of the protection of modified and unmodified dsDNA by cationic and non-cationic lipids and liposomes to digestion by DNase I. J Chem Soc Perkin Trans 2. 1998(6):1283–6.
Dass CR, Walker TL, Decruz EE, Burton MA. Cationic liposomes and gene therapy for solid tumors. Drug Deliv. 1997;4(3):151–65.
Saffari M, Moghimi HR, Dass CR. Barriers to liposomal gene delivery: from application site to the target. Iran J Pharm Res IJPR. 2016;15(Suppl):3.
Lee J-H, Lee M-J. Liposome-mediated cancer gene therapy: clinical trials and their lessons to stem cell therapy. Bull Korean Chem Soc. 2012;33(2):433–42.
Lai I, Swaminathan S, Baylot V, Mosley A, Dhanasekaran R, Gabay M, et al. Lipid nanoparticles that deliver IL-12 messenger RNA suppress tumorigenesis in MYC oncogene-driven hepatocellular carcinoma. J Immunother Cancer. 2018;6(1):1–11.
Luheshi N, Hewitt S, Garcon F, Burke S, Watkins A, Arnold K, et al. MEDI1191, a novel IL-12 mRNA therapy for intratumoral injection to promote TH1 transformation of the patient tumor microenvironment. AACR. 2019.
Charoensit P, Kawakami S, Higuchi Y, Yamashita F, Hashida M. Enhanced growth inhibition of metastatic lung tumors by intravenous injection of ATRA-cationic liposome/IL-12 pDNA complexes in mice. Cancer Gene Ther. 2010;17(7):512–22.
van der Heijden I, Beijnen JH, Nuijen B. Long term stability of lyophilized plasmid DNA pDERMATT. Int J Pharm. 2013;453(2):648–50.
Poxon SW, Hughes JA. The effect of lyophilization on plasmid DNA activity. Pharm Dev Technol. 2000;5(1):115–22.
Saha B, Saha D, Niyogi S, Bal M. A new method of plasmid DNA preparation by sucrose-mediated detergent lysis from Escherichia coli (gram-negative) and Staphylococcus aureus (gram-positive). Anal Biochem. 1989;176(2):344–9.
Mugabe C, Azghani AO, Omri A. Preparation and characterization of dehydration–rehydration vesicles loaded with aminoglycoside and macrolide antibiotics. Int J Pharm. 2006;307(2):244–50.
Wheeler J, Palmer L, Ossanlou M, MacLachlan I, Graham R, Zhang Y, et al. Stabilized plasmid-lipid particles: construction and characterization. Gene Ther. 1999;6(2):271.
Li S-D, Huang L. Targeted delivery of antisense oligodeoxynucleotide and small interference RNA into lung cancer cells. Mole Pharm. 2006;3(5):579–88.
Stuart D, Allen T. A new liposomal formulation for antisense oligodeoxynucleotides with small size, high incorporation efficiency and good stability. Biochim Biophys Acta Biomembr. 2000;1463(2):219–29.
Monsky WL, Fukumura D, Gohongi T, Ancukiewcz M, Weich HA, Torchilin VP, et al. Augmentation of transvascular transport of macromolecules and nanoparticles in tumors using vascular endothelial growth factor. Cancer Res. 1999;59(16):4129–35.
Cagdas FM, Ertugral N, Bucak S, Atay NZ. Effect of preparation method and cholesterol on drug encapsulation studies by phospholipid liposomes. Pharm Dev Technol. 2011;16(4):408–14.
Liang X, Mao G, Ng KS. Mechanical properties and stability measurement of cholesterol-containing liposome on mica by atomic force microscopy. J Colloid Interface Sci. 2004;278(1):53–62.
Shaker S, Gardouh AR, Ghorab MM. Factors affecting liposomes particle size prepared by ethanol injection method. Res Pharm Sci. 2017;12(5):346.
Wieber A, Selzer T, Kreuter J. Physico-chemical characterisation of cationic DOTAP liposomes as drug delivery system for a hydrophilic decapeptide before and after freeze-drying. Eur J Pharm Biopharm. 2012;80(2):358–67.
Li J, Wang X, Zhang T, Wang C, Huang Z, Luo X, et al. A review on phospholipids and their main applications in drug delivery systems. Asian J Pharm Sci. 2015;10(2):81–98.
Heurtault B, Saulnier P, Pech B, Proust J-E, Benoit J-P. Physico-chemical stability of colloidal lipid particles. Biomaterials. 2003;24(23):4283–300.
Tao J, Ding W-F, Che X-H, Chen Y-C, Chen F, Chen X-D, et al. Optimization of a cationic liposome-based gene delivery system for the application of miR-145 in anticancer therapeutics. Int J Mol Med. 2016;37(5):1345–54.
Li S, Rizzo M, Bhattacharya S, Huang L. Characterization of cationic lipid-protamine–DNA (LPD) complexes for intravenous gene delivery. Gene Ther. 1998;5(7):930.
Brgles M, Šantak M, Halassy B, Forcic D, Tomašić J. Influence of charge ratio of liposome/DNA complexes on their size after extrusion and transfection efficiency. Int J Nanomed. 2012;7:393.
O’Hara RJ, Greenman J, MacDonald AW, Gaskell KM, Topping KP, Duthie GS, et al. Advanced colorectal cancer is associated with impaired interleukin 12 and enhanced interleukin 10 production. Clin Cancer Res. 1998;4(8):1943–8.
Brunda MJ, Luistro L, Rumennik L, Wright RB, Dvorozniak M, Aglione A, et al. Antitumor activity of interleukin 12 in preclinical models. Cancer Chemother Pharmacol. 1996;38(1):S16–21.
Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13(2):155–68.
A Engel M, F Neurath M. Anticancer properties of the IL-12 family-focus on colorectal cancer. Curr Med Chem. 2010;17(29):3303-8.
Tugues S, Burkhard S, Ohs I, Vrohlings M, Nussbaum K, Vom Berg J, et al. New insights into IL-12-mediated tumor suppression. Cell Death Differ. 2015;22(2):237–46.
Jung K, Ha J-H, Kim J-E, Kim J-A, Kim Y-J, Kim C-H, et al. Heterodimeric Fc-fused IL12 shows potent antitumor activity by generating memory CD8+ T cells. Oncoimmunology. 2018;7(7):e1438800.
Somiari S, Glasspool-Malone J, Drabick JJ, Gilbert RA, Heller R, Jaroszeski MJ, et al. Theory and in vivo application of electroporative gene delivery. Mol Ther. 2000;2(3):178–87.
Wang J, Meng F, Kim B-K, Ke X, Yeo Y. In vitro and in vivo difference in gene delivery by lithocholic acid-polyethyleneimine conjugate. Biomaterials. 2019;217:119296.
Suzuki R, Namai E, Oda Y, Nishiie N, Otake S, Koshima R, et al. Cancer gene therapy by IL-12 gene delivery using liposomal bubbles and tumoral ultrasound exposure. J Control Release. 2010;142(2):245–50.
Maheshwari A, Mahato RI, McGregor J, Han SO, Samlowski WE, Park J-S, et al. Soluble biodegradable polymer-based cytokine gene delivery for cancer treatment. Molecular Ther. 2000;2(2):121–30.
Mahato RI, Lee M, Han SO, Maheshwari A, Kim SW. Intratumoral delivery of p2CMVmIL-12 using water-soluble lipopolymers. Mol Ther. 2001;4(2):130–8.
Kim TH, Jin H, Kim HW, Cho M-H, Cho CS. Mannosylated chitosan nanoparticle–based cytokine gene therapy suppressed cancer growth in BALB/c mice bearing CT-26 carcinoma cells. Mol Cancer Ther. 2006;5(7):1723–32.
Deshpande PP, Biswas S, Torchilin VP. Current trends in the use of liposomes for tumor targeting. Nanomedicine. 2013;8(9):1509–28.
Parayath N, Stephan S, Koehne A, Nelson P, Stephan M. In vitro-transcribed antigen receptor mRNA nanocarriers for transient expression in circulating T cells in vivo. Nat Commun. 2020;11(1):1–17.
Reinhard K, Rengstl B, Oehm P, Michel K, Billmeier A, Hayduk N, et al. An RNA vaccine drives expansion and efficacy of claudin-CAR-T cells against solid tumors. Science. 2020;367(6476):446–53.
Funding
This work was supported by Drug Applied Research Center and Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran.
Author information
Authors and Affiliations
Contributions
Somayeh Hallaj-Nezhadi is the supervisor and writes the paper and analyzes the data. Ali Mohammadi, Sara Shamekhi, Nikoo Majidazar, and Saiedeh Razi Soofiyani did all of the experiments. Behzad Baradaran, Nigel AJ McMillan, Farzaneh Lotfipour, and Azita Dilmaghani participated in the design of some of the experiments and writing the paper.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by Ethics Committee of Tabriz University of Medical Sciences, Tabriz, Iran (IR.TBZMED.VCR.REC.1398.051). The authors have participated in the preparation of the manuscript; they have given their permission to submit this manuscript to your journal.
Consent for publication
By signing this agreement, the author grants the publisher permission to publish the work named herein.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Baradaran, B., Mohammadi, A., Shamekhi, S. et al. A novel method for the development of plasmid DNA-loaded nanoliposomes for cancer gene therapy. Drug Deliv. and Transl. Res. 12, 1508–1520 (2022). https://doi.org/10.1007/s13346-021-01034-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-021-01034-0