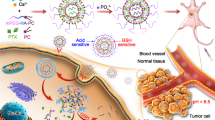
Abstract
At present, treating of triple negative breast cancer (TNBC) mainly depends on chemotherapy with more toxic side effects, but the effect is limited and it is highly prone to drug resistance. Gene therapy using anti-microRNAs maybe one of alternative therapeutic strategies. Due to the poor cell permeability and significant in vivo decomposition rate of anti-microRNAs, which limits their clinical application, we developed a core-shell supramolecular nanovector of “chitosome” that were self-assembled from the synthetic amphiphilic chitosan derivatives. The constructed chitosomes could co-load hydrophilic anti-miR-21 and hydrophobic docetaxel (DTX) into one combo nanocarrier with entrapment efficiency of more than 80%, as well as spherical morphology and average particle size of 90 nm. In comparison with the naked ones, anti-miR-21 encapsulated with chitosomes showed significantly increased cellular transfection and stability against degradation by nuclease in serum. Compared with DTX or anti-miR-21 formulations used alone, the co-delivery of the two drugs with the combo chitosome obtained improved chemosensitivity of TNBC cells to DTX treatment through their synergistic mechanisms. Taken together, the developed chitosome could be a promising candidate for simultaneous delivery of insoluble chemotherapeutic drugs and gene agents for TNBC therapy.

Graphical abstract









Similar content being viewed by others
References
Schmadeka R, Harmon BE, Singh M. Triple-negative breast carcinoma: current and emerging concepts. Am J Clin Pathol. 2014;141:462–77.
Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13:674–90.
Wahba HA, El-Hadaad HA. Current approaches in treatment of triple-negative breast cancer. Cancer Biol Med. 2015;12:106–16.
Kalimutho M, Parsons K, Mittal D, López JA, Srihari S, Khanna KK. Trends. Pharmacol Sci. 2015;36:822–46.
O'Toole SA, Beith JM, Millar EK, West R, McLean A, Cazet A, et al. Therapeutic targets in triple negative breast cancer. J Clin Pathol. 2013;66:530–42.
Mayer IA, Abramson VG, Lehmann BD, Pietenpol JA. New strategies for triple-negative breast cancer--deciphering the heterogeneity. Clin Cancer Res. 2014;20:782–90.
Kalimutho M, Parsons K, Mittal D, Lopez JA, Srihari S, Khanna KK. Targeted therapies for triple-negative breast cancer: combating a stubborn disease. Trends Pharmacol Sci. 2015;36:822–46.
Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer. 2015;15:321–33.
Krek A, Grun D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, et al. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495–500.
Cheng CJ, Bahal R, Babar IA, Pincus Z, Barrera F, Liu C, et al. MicroRNA silencing for cancer therapy targeted to the tumour microenvironment. Nature. 2015;518:107–10.
Yan LX, Huang XF, Shao Q, Huang MY, Deng L, Wu QL, et al. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA. 2008;14:2348–60.
Costa PM, Cardoso AL, Custodia C, Cunha P, Pereira de Almeida L, Pedroso de Lima MC. MiRNA-21 silencing mediated by tumor-targeted nanoparticles combined with sunitinib: a new multimodal gene therapy approach for glioblastoma. J Control Release. 2015;207:31–9.
Pereira DM, Rodrigues PM, Borralho PM, Rodrigues CM. Delivering the promise of miRNA cancer therapeutics. Drug Discov Today. 2013;18:282–9.
Li Z, Rana TM. Therapeutic targeting of microRNAs: current status and future challenges. Nat Rev Drug Discov. 2014;13:622–38.
Ben-Shushan D, Markovsky E, Gibori H, Tiram G, Scomparin A, Satchi-Fainaro R. Overcoming obstacles in microRNA delivery towards improved cancer therapy. Drug Deliv Transl Res. 2014;4:38–49.
Godbey WT, Barry MA, Saggau P, Wu KK, Mikos AG. Poly(ethylenimine)-mediated transfection: a new paradigm for gene delivery. J Biomed Mater Res. 2000;51:321–8.
Safinya CR, Ewert KK, Majzoub RN, Leal C. Cationic liposome-nucleic acid complexes for gene delivery and gene silencing. New J Chem. 2014;38:5164–72.
Ozpolat B, Sood AK, Lopez-Berestein G. Liposomal siRNA nanocarriers for cancer therapy. Adv Drug Deliv Rev. 2014;66:110–6.
Ragelle H, Riva R, Vandermeulen G, Naeye B, Pourcelle V, Le Duff CS. Chitosan nanoparticles for siRNA delivery: optimizing formulation to increase stability and efficiency. J Control Release. 2014;176:54–63.
Buschmann MD, Merzouki A, Lavertu M, Thibault M, Jean M, Darras V. Chitosans for delivery of nucleic acids. Adv Drug Deliv Rev. 2013;65:1234–70.
Chiu YL, Ho YC, Chen YM, Peng SF, Ke CJ, Chen KJ. The characteristics, cellular uptake and intracellular trafficking of nanoparticles made of hydrophobically-modified chitosan. J Control Release. 2010;146:152–9.
Liu CG, Desai KG, Chen XG, Park HJ. Linolenic acid-modified chitosan for formation of self-assembled nanoparticles. J Agric Food Chem. 2005;53:437–41.
Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–6.
Orellano MS, Porporatto C, Silber JJ, Falcone RD, Correa NM. AOT reverse micelles as versatile reaction media for chitosan nanoparticles synthesis. Carbohydr Polym. 2017;171:85–93.
Budker VG, Slattum PM, Monahan SD, Wolff JA. Entrapment and condensation of DNA in neutral reverse micelles. Biophys J. 2002;82:1570–9.
Fishburn CS. The pharmacology of PEGylation: balancing PD with PK to generate novel therapeutics. J Pharm Sci. 2008;97:4167–83.
Zhang W, Cheng Q, Guo S, Lin D, Huang P, Liu J, et al. Gene transfection efficacy and biocompatibility of polycation/DNA complexes coated with enzyme degradable PEGylated hyaluronic acid. Biomaterials. 2013;34:6495–503.
Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99:28–51.
Fang Y, Xue J, Gao S, Lu A, Yang D, Jiang H, et al. Cleavable PEGylation: a strategy for overcoming the "PEG dilemma" in efficient drug delivery. Drug Deliv. 2017;24:22–32.
Coulthard LG, Woodruff TM. Is the complement activation product C3a a proinflammatory molecule? Re-evaluating the evidence and the myth. J Immunol. 2015;194:3542–8.
Minami S, Suzuki H, Okamoto Y, Fujinaga T, Shigemasa Y. Chitin and chitosan activate complement via the alternative pathway. Carbohydr Polym. 1998;36:151–5.
Cambon A, Figueroa-Ochoa E, Juarez J, Villar-Alvarez E, Pardo A, Barbosa S, et al. Complex self-assembly of reverse poly(butylene oxide)-poly(ethylene oxide)-poly(butylene oxide) triblock copolymers with long hydrophobic and extremely lengthy hydrophilic blocks. J Phys Chem B. 2014;118:5258–69.
Xiang S, Tong H, Shi Q, Fernandes JC, Jin T, Dai K, et al. Uptake mechanisms of non-viral gene delivery. J Control Release. 2012;158:371–8.
Lonn P, Kacsinta AD, Cui XS, Hamil AS, Kaulich M, Gogoi K, et al. Enhancing endosomal escape for intracellular delivery of macromolecular biologic therapeutics. Sci Rep. 2016;6:32301.
Mao S, Sun W, Kissel T. Chitosan-based formulations for delivery of DNA and siRNA. Adv Drug Deliv Rev. 2010;62:12–27.
Duceppe N, Tabrizian M. Advances in using chitosan-based nanoparticles for in vitro and in vivo drug and gene delivery. Expert Opin Drug Deliv. 2010;7:1191–207.
Ghanbari Safari M, Hosseinkhani S. Lipid composition of cationic nanoliposomes implicate on transfection efficiency. J Liposome Res. 2013;23:174–86.
Hsu CY, Uludag H. Cellular uptake pathways of lipid-modified cationic polymers in gene delivery to primary cells. Biomaterials. 2012;33:7834–48.
Di Leva G, Croce CM. miRNA profiling of cancer. Curr Opin Genet Dev. 2013);23:3–11.
Zhang BG, Li JF, Yu BQ, Zhu ZG, Liu BY, Yan M. microRNA-21 promotes tumor proliferation and invasion in gastric cancer by targeting PTEN. Oncol Rep. 2012;27:1019–26.
Fang H, Xie J, Zhang M, Zhao Z, Wan Y, Yao Y. miRNA-21 promotes proliferation and invasion of triple-negative breast cancer cells through targeting PTEN. Am J Transl Res. 2017;9:953–61.
Mayer IA, Arteaga CL. The PI3K/AKT pathway as a target for cancer treatment. Annu Rev Med. 2016;67:11–28.
Abdel-Fatah TM, Perry C, Dickinson P, Ball G, Moseley P, Madhusudan S, et al. Bcl2 is an independent prognostic marker of triple negative breast cancer (TNBC) and predicts response to anthracycline combination (ATC) chemotherapy (CT) in adjuvant and neoadjuvant settings. Ann Oncol. 2013;24:2801–7.
Sharifi S, Barar J, Hejazi MS, Samadi N. Roles of the Bcl-2/Bax ratio, caspase-8 and 9 in resistance of breast cancer cells to paclitaxel. Asian Pac J Cancer Prev. 2014;15:8617–22.
Funding
This research was funded by the Basic and Frontier Technology Research Projects from Science and Technology Department of Henan Province (No. 142300410271, the screening and analysis of specific microRNA expression in peripheral blood of patients with bone metastasis after breast cancer surgery).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, X., Xu, H., Huang, T. et al. Simultaneous delivery of anti-miRNA and docetaxel with supramolecular self-assembled “chitosome” for improving chemosensitivity of triple negative breast cancer cells. Drug Deliv. and Transl. Res. 11, 192–204 (2021). https://doi.org/10.1007/s13346-020-00779-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-020-00779-4